Introduction
Atrial fibrillation (AF), a cardiac rhythm disorder, predisposes to significant morbidity and mortality due to thromboembolism and stroke. There is an increased risk of stroke and stroke related deaths in these patients, especially in presence of other comorbidities and risk factors for stroke.1
Anticoagulant therapy helps reduce the risk of ischemic stroke in patients with AF. The risk of stroke and incidence of death significantly reduced with the vitamin-Kantagonist (VKAs) anticoagulants. However, these drugs predispose to increased risk of intracranial hemorrhage (ICH) and major bleeding episodes. The NOACs demonstrated a similar efficacy with superior safety as compared to the VKAs.2 As per the recent guidelines, the NOACs are the preferred drugs for stroke prevention in patients with atrial fibrillation.3
Management of emergency procedures and bleeding with NOACs poses a challenge for the health care professionals. The availability of reversal agents for the NOACs might provide as an option for the management in such cases. However, the reversal agents for all NOACs are not available.4
Idarucizumab (Praxbind), a humanized monoclonal antibody, is the specific reversal agent for dabigatran (Pradaxa), a direct thrombin inhibitor. The binding affinity to dabigatran is 350 times higher as compared to the affinity of dabigatran to thrombin. The effects of dabigatran can be immediately and complete ly revers ed with the administration of idarucizumab. No major safety related issues have been reported with the use of idarucizumab.5 Apart from binding Dabigatran, idarucizumab also forms irreversible 1:1 stoich iometric complexes with the glucuronide metabolites of dabigatran.6
The re is limited evidence for the use of idarucizumab in India. Mashru et al reported a case of use of Idarucizumab in a patient on treatment with dabigatran for management of intracranial hemorrhage.7 W e report two cases of successful use of Idarucizumab in patient s of atrial fibrillation on treatment with dabigatran, one with acute ischemic stroke and the other with intracranial hemorrhage.
Case Description
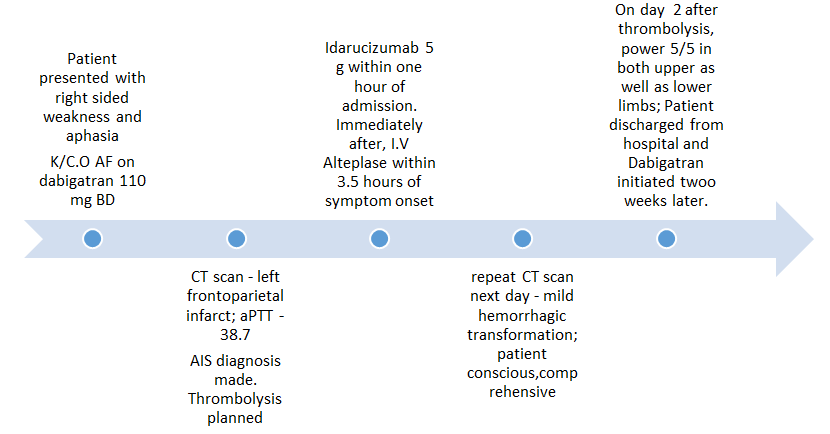
Case 1 – A case of acute ischemic stroke Figure 1
A 49 years old female patient presented to our hospital with complaints of a sudden onset weakness in the right upper and lower limbs with aphasia, with the onset around 30 minutes prior to presentation. The patient also had diabetes mellitus and hypertension. She had atrial fibrillation and was taking tablet dabigatran 110 mg twice daily. The patient had last taken dabigatran on the day of admission.
At presentation, the comprehension and naming ability of the patient were impaired. Neurological examination depicted a right upper limb power of 1/5 and right lower limb with power 3/5. National Institutes of Health Stroke Scale (NIHSS)8 score was 20.
The patient was normotensive at the time of presentation (BP-130/80mm Hg). Laboratory profile of the patient demonstrated an elevated activated partial thromboplastin time (aPTT) of 38.7 seconds (N=15-30s). A computed tomographic analysis of the brain revealed a left sided fronto-parietal infarct with a normal angiographic picture (Report in Figure 2). A cute ischemic stroke was diagnosed and thrombolysis was planned.
Chart 1
CTA scan report at the time of admission depicting hyperacute infarct in left middle cerebral

Chart 2
Follow-up CT scan depicting infarct evolution in the left parietal lobe with minimal haemorhhagic transformation

Chart 5
MRI after 20 days after the incident event indicating resolving haemorrhage, the patient was restarted on Pradaxa after this follow up.

The patient was administered idarucizumab within 60 minutes of admission to the hospital. Following idarucizumab, i.v. alteplase was administered within three and half hours of symptom onset. A repeat CT scan on the next day demonstrated a mild haemorrhagic transformation (Report In Figure 3). However, the patient was conscious, comprehensive and was able to speak. On the second day after thrombolysis, the neurological examination of the patient depicted a 5/5 power in both the right upper as well as the lower limb. The patient was subsequently discharged from the hospital and dabigatran was restarted after two weeks of the said episode.
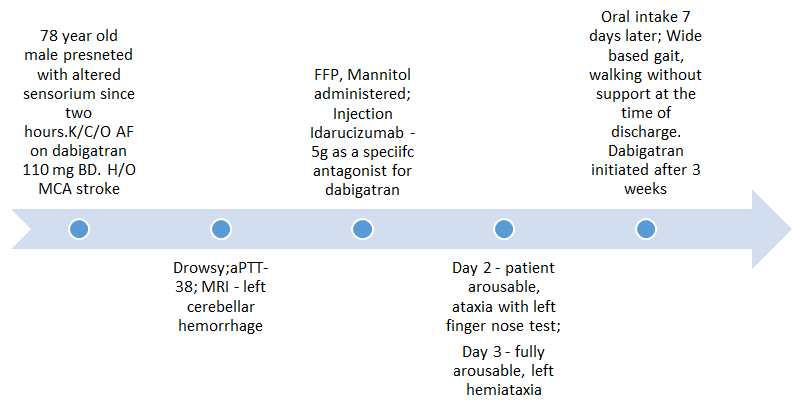
Case report 2 – A case of intracranial haemorrhageFigure 4
A 78 year old male patient of atrial fibrillation, was being treated wit h twice daily dosing of dabigatran 110 mg, and reported with the chief complaint of altered sensorium for the past two to three hours. The patient had a history of Middle Cerebral Artery (MCA) territory stroke, and was also being treated for hypothyroidism and diabetes mellitus.
The patient appeared drowsy on general examination. On neurological examination, the grip of the left hand was weak and the left upper and lower limb power was observed to be 4/5.
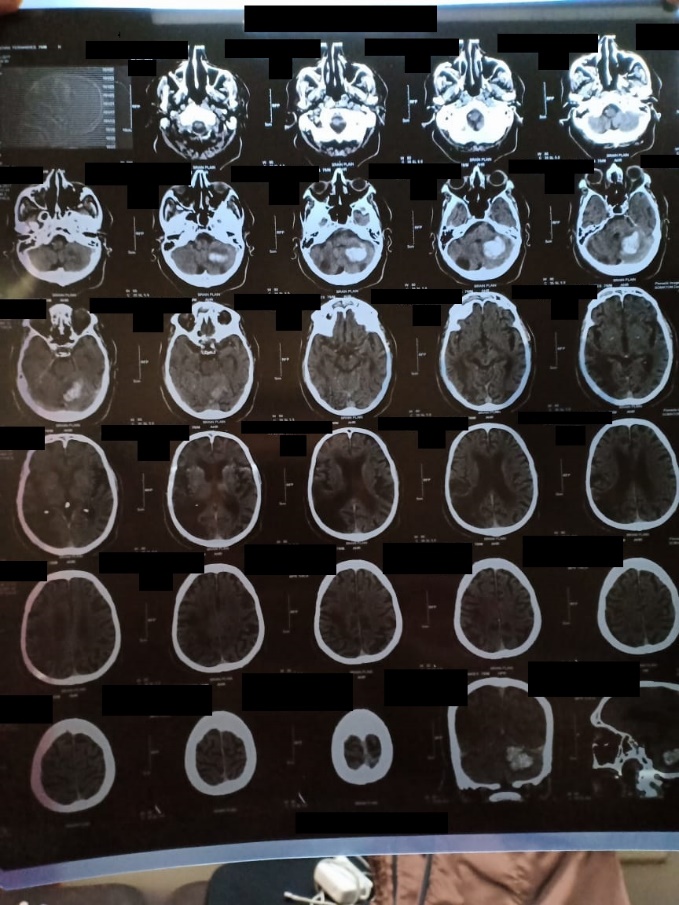
The clinical parameters were normal. The laboratory profile of the patient depicted a raised aPTT =38 seconds [Normal range (N)=15-30 s]. A magnetic resonance imaging (MRI) report was obtained and it demonstrated a left cerebellar haemorrhage of size 4.5×3 cm Figure 5.
The patient was administered fresh frozen plasma and intravenous mannitol. The specific antagonist for dabigatran, Idarucizumab (5g) was also administered. The patient was fed using a Ryle’s tube.
The patient was rousable and the pupils were reactive to light, the day after idarucizumab administration. Ataxia was observed during the left finger nose test. On the 3rd day after idarucizumab treatment, patient was fully rousable with normal comprehension but with left hemiataxia. The recovery was slow and the patient was gradually mobilized. The patient started oral intake of food after seven days. The patient had a wide based gait and walked without support at the time of discharge Figure 6. Treatment with dabigatran was initiated three weeks after patient recovery Figure 7.Discussion
The NOACs are approved as first line agents for stroke prevention in atrial fibrillation. A rapid onset/offset of action, paucity of drug interactions, and a specific reversal agent in the case of dabigatran provide a benefit for the use of NOACs in such patients.9
Thrombolysis using r-tPA is the treatment for patients suffering from an acute ischemic stroke. The concomitant use of anticoagulants is a contraindication for the use of r- tPA. Thrombolysis can be initiated in such patients based on the recent medication history or no clinically relevant anticoagulant effects as demonstrated by confirmatory laboratory investigations. Alternatively, a specific reversal agent can be used to reverse the anticoagulant effect of the drug and thrombolysis with r- tPA can be undertaken.10,11 In our patient with acute ischemic stroke, the aPTT was prolonged indicating the anticoagulant effect of dabigatran was still present. Idarucizumab 5g was used before initiating thrombolysis in the patient to attain successful dabigatran reversal.
ICH is the most common neurological complication associated with the use of oral anticoagulants. Thus it poses a major concern with respect to initiation of treatment with NOACs, especially in the elderly population. ICH can be intracerebral, subdural or subarachnoid bleeding.12 Management of ICH is a challenging prospect. Pharmacotherapy with fresh frozen plasma (FFP), prothrombin complex concentrates (PCCs), and phytonadione aim at reducing hematoma expansion, midline shift and the related mass effect. Mannitol also helps in controlling the edema. Neurosurgery and neuro -inten sivist intervention is required in uncontrollable cases or cases with a major neurological deficit. If the patient on a NOAC is conscious with no major residual defect and no comorbidity, surgery can be deferred and the normal excretion of the NOAC allowed due to their shorter half life. Specific reversal agent helps to attain prompt reversal of the action of Dabigatran. Unlike warfarin, the reversal agent for dabigatran demonstrates a prompt reversal effect.13 Our patient presented with complaints of altered sensorium for a duration of 2-3 hours and had a minimal deficit as depicted with power 4/5 on neurological examination. The patient was managed using fresh frozen plasma, mannitol and idarucizumab. The patient demonstrated a gradual recovery from the symptoms after administration of idarucizumab. As a result, surgical intervention was deferred for this patient. Another case report of ICH in a patient on dabigatran also described recover y with the use of idarucizumab without surgical intervention.7
A review of literature highlights the reversal of anticoagulation with dabigatran using Idarucizumab in patients requiring emergency surgeries or suffering from major bleeding. Reversal of anticoagulant effect was seen within a very short period of time in patients with both the interventions.5
Conclusion
Idarucizumab helps reverse the anticoagulation with dabigatran in patients presenting with bleeding as well as patients undergoing thrombolysis for treatment of acute ischemic stroke. The outcomes in both cases were uneventful and the patients were restarted on dabigatran therapy within four weeks of the respective episodes.