Introduction
Neurocysticercosis (NCC) is a clinical condition characterized by the parasitic involvem ent of the central nervous system by the larval stage of the pork tapeworm Taenia solium and has been estimated to cause 50,000 deaths worldwide annually.1 In India itself, NCC has been identified as a cause of 2.2 to 6% unselected cases of seizures.2
Several varieties of NCC have been recognized depending upon the number, location, and evolutionary stage of the cysterci in the human brain. Solitary cysticercus granuloma (SCG) which in essence represents a degenerating single cerebral cysticercus cyst,3 is a common presenting form of NCC particularly in India and the United States,4 and may cause recurrent seizures difficult to control with multiple anti-epileptic drugs.5
Because of the epidemiologic importance of SCG, difficulty in the predicting the outcome and the pivotal role of magnetic resonance imaging (MRI) in its evaluation, it is of paramount importance to determine an optimal MR imaging prognostic protocol when scanning patients with SCG.6 A dvanced MRI techniques have recently been used in this context to further study the characteristics of these lesions,7,8,9 but there are scant studies comparing the ability of prognostication of MRI imaging in SCG and then studying its effect on the outcome of resolution.
This pilot study aimed to study selected conventional and advanced MR imaging parameters as prognostic markers in the outcomes of resolution of SCG.
Materials and Methods
This was a longitudinal, prospective observational study conducted after approval of the institutional research and ethics committee, over a period of one year from March 2013 to March 2014, and included 40 patients. The cases included were detected and diagnosed both in the OPD and IPD of the Neurology department.
Inclusion criteria
Patients consistent with a diagnosis of SCG as per the revised clinical and radiological (MRI) diagnostic criteria for SCG were included in this study.6 Table 1
Exclusion criteria
Patients of tuberculosis, known malignancy, renal failure or symptomatic secondary epilepsies as well as HIV-reactive patients were excluded due to a greater chance of intracranial space-occupying lesions in these patients.
A detailed clinical history regarding the onset of symptoms and clinical progression of the disease process was taken along with special consideration to general and neurological examination. They then were subjected to conventional MR imaging, fluid attenuated inversion sequence (FLAIR), diffusion weighted imaging (DWI), proton magnetic resonance spectroscopy (MRS) and magnetization transfer MRI (MT - T1 and T2 weighted); with contrast enhanced T1 weighted sequences done wherever indicated. Informed consent was obtained from all the subjects/guardians before the study.
Number, site, stage, size of the lesions, presence of scolex, grade of perilesional oedema10, restriction on DWI, ADC values11, type of enhancement, levels of various neurotransmitters on MRS, magnetization transfer ratio (MTR), and presence of perilesional gliosis (on MT sequence) were noted. Patients were followed up regularly for six months after inclusion into the study, when MRI Brain was repeated and degree of resolution as well as calcification, if any, were noted. Radiological resolution was taken as complete resolution or partial resolution or calcification without oedema at the end of six months.
All patients received albendazole in a dose of 15 mg/kg body weight/day in 2–3 divided doses for one to two weeks and oral prednisolone 2 mg/kg/day for initial 5 days of therapy. Antiepileptic drugs were given for the period of the study.
Following were considered indicators of therapeutic outcome:
Radiological outcome
Complete resolution (CR) - Disappearance of lesion.
Partial resolution (PR) - Reduction in size of lesion by at least 50% or more of the original.
No resolution (NR) - No or less than 50% decrease in the size of the original lesion.
Calcification of the lesion - appearance of small calcified speck without oedema.
Clinical outcome w as measured in terms of recurrence of seizures, based on objective reports from patients.
The role of MRI techniques in predicting the outcome of S CG and the correlation of radiological outcome with recurrence of seizures was analyzed using standard statistical methods (descriptive statistics, Chi square test, Student ’ st-test, Analysis of variance test [ANOVA test] and Kruskal-Wallis test).
Results
Patient characteristics
There were 40 patients in the study with a mean age of 25.93 ± 15.22 years and median age of 20.5 years. Most of them belonged to the age group of <20 years (50%), followed by 21 to 40 years (35%) and >40 years (15%). Among all, 24(60%) were males and 16(40%) were females with male: female ratio being 1.5. Half of them were pure vegetarians. None of the remaining was a pork-eater and no subject had domestic or occupational exposure to pigs.
Characteristics of first MRI scan
97.5% of patients had supratentorial involvement (commonest being parietal lobe); whereas only one patient had infratentorial lesion. In the present study, granular nodular stage was encountered in 32(80%) of the total 40 subjects p resenting with solitary lesions. Of the 40 patients, 36 (90%) patients were found to have lesions ≤10mm, whereas 4(10%) had lesions ≥10mm.
Thirty-six (90%) patients had perilesional oedema at the time of presentation. 25( 62.5%) of the patients had perilesional oedema of ≤ grade 2 (less than twice the size of lesion) while it was > grade 2 in 11 (27.5%) of the patients.10 None of the patients had any mass effect due to the lesion. Presence of scolex was seen in 45% patients. Diffusion restriction was observed in 7.5% of patients. Mean ADC values from the core, the scolex and the wall of the SCG lesions were (1.493 ± 0.252) X 10-3 mm2/s, (1.200 ± 0.487) X 10-3 and (1.335 ± 0.207) X 10-3 mm2 /s respectively. Majority of the ADC values from the scolex (50%), core (60%) and the wall (71.43%) were found to lie in category 3 i.e. ADC higher than normal white matter (ADC ranges: more than 1.05 X 10-3 mm2 /s).11
MR spectroscopy was done in 15 out of 40 patients. Levels of metabolites as N-Acetyl aspartate (NAA), Choline (Cho) and Creatine (Cr) were lower than normal brain parenchyma with values of 6.48±5.28 (control: 8.97± 4.23), 4.83±1.51 (control: 5.44±2.54) and 4.45 ± 2.04 (control: 6.62±4.22) respectively; the result reaching statistical significance for Cr (p=0.042) and near significan t for NAA (p=0.083). Lactate peak was seen in 66.7% of cases. Mean normalized Cho/Cr, NAA/Cr and NAA/Cho ratios were1.298±1.28 (control: 1.09±0.61), 1.41±0.81 (control: 1.54±1.09) and 1.63±1.74 (control: 2.68±2.24) respectively with median values of the same being 0.95 (control: 1.125), 1.22 (control: 1.8) and 1.14 (control: 2.02) respectively. No statistical significance was found.
MT imaging was done in 11 out of 40 cases. MTR values from the lesion and perilesional area were found to be 10.53 ± 4.5 and 11.28 ± 3.98 (control: 14.55 ± 5.62) for MT T1 images and 13.92 ± 11.6 and 8.91±9.97 (control: 11.9 ± 11) for MT T2 images. T1 MTR values in the lesion were found to be significantly lower (p = 0.0396) than the controls, whereas in the case of perilesional areas it was near significant (p = 0.0651).
Clinical outcome
Clinical outcome w as assessed after six months on the basis of recurrence of seizures, based on objective reports from thirty seven patients; three subjects being lost to follow-up. Twenty-eight (75.68%) patients were free of seizures; while nine patients (24.32%) had recurrence of seizures at the end of six months.
Of the 28 patients who were seizure free, 8(28.57%) had complete resolution of the lesion on follow up MRI scan at the end of six months, 17 (60.71%) had partial resolution; with lesions remaining unaltered in 3 (10.71%) patients.
And of the nine patients who had a recurrence of seizure during this period, 22.2% had no resolution in their lesions, with only partial resolution in the rest 77.8% of the patients. No subject with recurrent seizures showed a complete resolution of the SCG lesion.
According to resolution of lesions, 40%(2/5) patients in the no-resolution and 29.2%(7/24) in the partial resolution had recurrence of seizure activity; while no recurrent seizures were reported in the complete resolution group. No statistical significance was found.
Radiological outcome
21.6% patients showed complete resolution and 64.9% partial resolution, with 13.5% showing no resolution in the lesions on follow up MRI scan after an interval of 6 months. No subject showed appearance of any new lesion or any calcification in the previous cysticercus. Various conventional parameters like age, gender, size of lesion, grade of perilesional oedema, presence or absence of scolex, signal intensity on T1W and T2W images, pattern of enhancement, presence or absence of diffusion restriction on DW images were not found to be helpful in predicting the resolution of the lesions in SCG patients. There was also no statistical correlation between the ADC values of core, wall or scolex as well as MTR values and resolution of SCG lesions.
Median normalized Cho/Cr ratios were 5.910 in the complete resolution (CR) group as compared to 0.925 in the no resolution (NR) group, with the result being statically significant (p < 0.001). Similarly the median normalized NAA/ Cr ratios were 3.420 and 1.280 in the CR and the NR groups respectively (p < 0.001). Normalized NAA/Cho ratios (median values) of 0.58 and 1.60 for the CR and NR groups respectively, though not statistically significant, did reveal a positive trend of NAA/Cho <1 favouring resolution. Figure 1
Perilesional gliosis was seen in 18.2% cases in whom MT imaging was done; with none of the patients showing any resolution in their lesions. 50% of these had recurrent seizures as com pared to only 14% in the patients without any perilesional gliosis. No statistical significance was found.
Discussion
SCG are the most common graunulomatous lesions of the brain in our country, for the localization of which MRI has emerged as the most valuable imaging modality. As the most important issue today relates to determining the resolution of the SCG lesions and also in predicting their outcome, a cause and effect relationship between MRI imaging parameters and epileptogenesis in patients with SCG is very essential for it is known that seizure activity is independent of the persistence or disappearance of the granuloma.12
NCC lesions showed a propensity for a younger population, 0 – 20 years (50%). Similar predisposition for younger patients has been reported by Garg and Nag (1997) who observed an incidence of 72% in children and adolescents.13
The most common locations reported for these lesions by Chandy (1991)14 are the Parietal lobes.15 In the present study also, most of the lesions (97.5%) were observed in the supratentorial location with 45% being confined to parietal lobe.
In the present study, granular nodular stage was encountered in 32(80%) of the total 40 subjects presenting with solitary lesions. This is comparable with the study of Rajshekhar et al (2003) who described Granular nodular form of parenchymatous cysts to account for 62–70% of all forms of NCC among Indian patients.16
Pretell et al (2005) has mentioned that cysticercosis can show wide areas of oedema. This was observed in the present study also.17
All 40 cases were less than 20 mm in our study. This compares with the study done by Rajshekhar et al (1993) in which all NCC lesions were less than 20 mm in size.18
In our study, the signal intensity of the cyst fluid was found to be higher than that of CSF on both T1W and T2W images. The surrounding oedema appeared as high intensity while cyst wall and central scolex when visualized (45% cases) appeared as a hyper intense dot on T1W and FLAIR images and as hypo intense dot on T2W sequence. Enhancement pattern was both ring shaped and nodular.
Follow up imaging was done at 6 months in our study. Partial resolution (PR) was seen in 64.87% of the patients and complete resolution was seen in 21.62% of the patients. Lesions persisted in 13.51% of the patients. Rajshekhar (2001) studied the rate of spontaneous resolution of freshly diagnosed SCG (imaging done within a month of onset of seizures) in 210 patients and found that only 18% of granulomas had resolved completely by 3 months.19
Singh et al (2001) in a prospective study of 75 patients with single granular nodular lesion on antiepileptic drug therapy found that the majority of patients (87%) remained seizure free after 1 year follow up examination.20
Diffusion Weighted Imaging
Diffusion restriction (DR) was seen in only three cases of SGC (7.5%), which corroborates with the findings of Santos et al (2012) who concluded that DR may be present in cystic parenchymal lesions in the colloidal vesicular and granular nodular phases.21 Thus, DW1 may signal a continuum of degeneration. As per the classification given by R.N. Senner (2001), majorit y of ADC values from the scolex, core and the wall of the lesions were found to lie in category 3 i.e. ADC higher than normal white matter (ADC ranges: more than 1.05 X 10-3 mm2 /s).11 Similar findings have been reported by Gupta et al (2005).22
MR Spectroscopy [Fig. 1]
MRS revealed alactate peak as well as lower concentration of metabolites NAA, Cho and Cr in the lesion as compared to normal areas. Sharda et al8 and Pandit et al23 also found similar results in NCC. Median normalized Cho/Cr, NAA/Cr and NAA/Cho ratios were 0.95 (control: 1.125), 1.22 (control: 1.8) and 1.14 (control: 2.02) respectively. Similar results were illustrated by Pretell et al.17
Magnetization Transfer MRI Figure 2
Perilesional gliosis was seen in two of the eleven patients (18.2%) of SCG in whom MT imaging was done. This finding corroborates with the findings of S. Pradhan et al (2000) who observed that SCG, albeit in small numbers, can be associated with perilesional gliosis and concluded that there is a positive relationship between perilesional gliosis and epileptogenesis in patients with healed/healing SCG. The authors observed gliosis in 22/108 (20.37%) patients with healing and healed SCG and persistent seizure activity was observed in 19/22 of these cases.12 Thus, MTR may signal a continuum of persistent epileptogenesis. MTR values from the lesion and perilesional area were found to be 10.53±4.5 and 11.28±3.98 (control: 14.55±5.62) for MT T1 images and 13.92±11.6 and 8.91±9.97 (control: 11.9 ± 11) for MT T2 images.
The major thrust of the present study was not only to characterize the individual imaging features of SGC, but also to analyze the usefulness of DW sequence, MR Spectroscopy, MT MRI sequences and other MRI parameters in determining the resolution of the SCG lesions and also in predicting the outcome of lesions. Apart from the relevant demographic characteristics, the following imaging features were analyzed: Table 2,Table 3
Site of lesion
Lesions in infratentorial location were seen in 2.5% cases of SGC. Complete resolution was noted in 22.2% of supratentorial lesions. None of the infratentorial lesions had complete resolution. The statistical correlation was significant (p=0.037).
No significant statistical difference was found with resolution of SCG according to T1 and T2 signal intensity, the grade of perilesional oedema, presence of scolex, diffusion restriction.
ADC values
ADC values from the core, the scolex and the wall were (1.493 ± 0.252) X 10-3 mm2 /s, (1.200 ± 0.487) X 10-3 and (1.335 ± 0.207) X 10-3 mm2 /s. As per the classification given by R.N. Senner (2001 )11, majority of ADC values from the scolex, core and the wall of the lesions were found to lie in category 3 i.e. ADC higher than normal white matter (ADC ranges: more than 1.05 X 10-3 mm2 /s). Similar findings have been reported by Gupta et al (2005).22 No significant statistical association of different grades of oedema with resolution of the lesions was found.
Table 1
Revised clinical and radiological diagnostic criteria for SCG6
[i] * Seizures may be new-onset or of longer duration, may be generalized at onset; may occur in clusters (2 or more seizures over 2–3 days); may be followed by unilateral or diffuse headaches lasting for a few hours to days; may or may be followed by transient and mild postictal neurologic deficits
Table 2
Role of demographic & conventional imaging characteristics in predicting the resolution of SCG*
Table 3
Role of advanced imaging characteristics in predicting the resolution of SCG*
[i] * excluding 3 patients lost to follow up † CR stands for complete resolution; PR for partial resolution; NR for no resolution ‡Pat. Stands for patients §measured in ppm; NAA stands for n-acetyl aspartate; Cho for choline; Cr for creatine ¶ by ANOVA method || L stands for lesion; PL for perilesional.
Table 4
| Seizure recurrence | SCG | Total no. (%) | p value | ||
| CR † | PR † | NR † | |||
| No | 8 (100) | 17(70.83) | 3 (60) | 28 (75.68) | 0.170 |
| Yes | 0 | 7(29.17) | 2 (40) | 9 (24.32) | |
Percentage of seizure recurrence in SCG according to resolution of lesions*
Table 5
| Total no. of cases (N=9 * ) | Persistent seizures | No seizures | p value |
| Gliosis (n=2) | 1(50%) | 1(50%) | 0.284 |
| No gliosis (n=7) | 1(14.3%) | 6(85.7%) |
Seizure recurrence in SCG according to perilesional gliosis
Figure 1
Right Parietal SCG with partial resolution; A. Granulomatous lesion with scolex with surrounding oedema; B. MRS showed low metabolite levels, as well as a lactate peak; C. Lower NAA/Cho ratio on MRS (arrow); 4. MRS depicting higher Cho/Cr ratio (arrow). Patient had partial resolution of the lesion on follow up imaging.
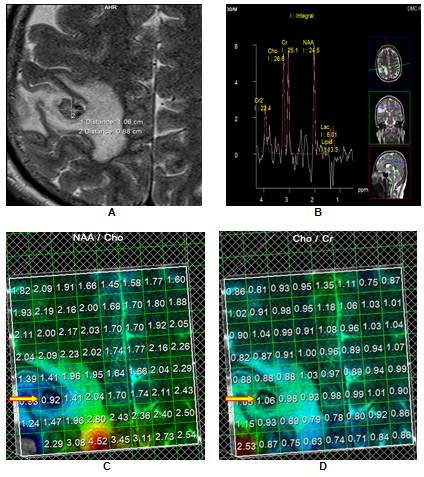
Figure 2
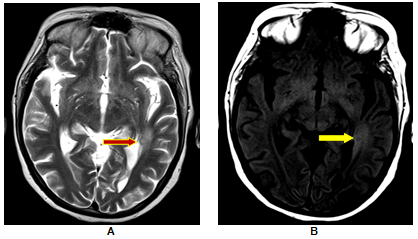
Left Temporal SCG with no resolution; A. T2W image shows a small hyperintense area and minimal perilesional oedema; B. The perilesional hyperintensity better demonstrated (yellow arrow) on T1-MT images is seen to extend beyond the abnormality (red arrow) seen on T2-W images and is suggestive of perilesional gliosis. Patient had no resolution of the lesion on follow up imaging.
MR Spectroscopy
Median normalized Cho/Cr ratios were 5.910 in the complete resolution (CR) group as compared to 0.925 in the no resolution (NR) group, with the result being stastically significant (p < 0.001). Similarly the median normalized NAA/Cr ratios were 3.420 and 1.280 in the complete resolution and the no resolution groups respectively (p < 0.001). Creatine (Cr) is an extremely reliable marker of intact brain metabolism.23 In the present case, the reduced Cr, at location 3.0ppm, reflected the absence of brain tissue within the lesion. Early degeneration of the SCG lesions in the CR group caused the metabolite levels to fall rapidly to lower levels than seen in the NR group; thereby accounting for the significantly higher Cho/Cr and NAA/Cr ratios in the that group.Figure 1
Normalized median NAA/Choline ratios of 0.58 and 1.60 for the CR and NR groups respectively, revealed a positive trend of NAA/Choline <1 favouring resolution.Figure 1 In the SCG, Choline (Cho) is elevated owing to the breakdown of phosphatidylcholine and the release of phosphorylcholine and glycerophosphorylcholine and NAA reduced on account of the absence of neurons and axons. Again early degeneration in the CR category helps in explaining the early peaking of choline levels with a rapid fall in NAA levels, resulting in a lower NAA/Choline ratio in the that group.23
Radiological resolution of SCG lesions & Clinical outcome [Table 4]
Seizure recurrence after first seizure was observed in 24.32%(9/37) of patients with SCG. According to resolution of lesions, 40%(2/5) patients in no-resolution group and 29.2%(7/24) patients in partial resolution group had recurrence of seizure activity. Data from several controlled trials suggested that seizures could recur in 13% – 48% of the individuals while on AEDs alone for 6–15 months.24,25,26 On the other hand, in a prospective, observational study evaluating the incidence of seizure recurrence following resolution of the granuloma and withdrawal of AEDs (mean follow-up period 66 months) in a referral hospital-based cohort of individuals, 15% of the individuals had seizures.5 It is now known that seizure activity is independent of the persistence or disappearance of the granuloma.12
Perilesional gliosis was seen in 18.2% cases of SCG. These patients had no resolution in their lesions with 50% reporting recurrence in seizure activity. At the same time absence of perilesional gliosis was associated with recurrent seizure activity in only 14% of the subjects.Table 5 This finding corroborates with the findings of S. Pradhan et al who observed that perilesional gliosis can be associated with SCG in small n umbers and concluded that there is a positive relationship between perilesional gliosis and epileptogenesis in patients with healed/healing NCC.12
To summarize, the clinical and radiological markers which helped us in characterizing SCG are focal seizures, a young age group (<40 years), well defined lesion < 20mm in size, supratentorial location, T1 sequence intensity hypo/ isointense to CSF, T2 sequence hyperintense with hypointense rim, ring enhancement after contrast, presence of scolex, Grade 3 perilesional oedema10 , no DR on DW images, ADC values from scolex, core and wall lying in category 311 , presence of lactate peak and absence of lipid peak with lower concentration of NAA, Cho and Cr, lower mean Cho/Cr and NAA/Cr ratios and low perilesional T1 and T2 MTR as compared to normal areas.
In our study, we found a direction of resolution for some variables as lower NAA/Cho and higher Cho/Cr as well as NAA/Cr ratios on MRS, absence of perilesional gliosis and absence of seizure recurrence; with higher NAA/Cr and Cho/Cr ratios on MRS in the resolution group reaching a statistical significance.
There are several limitations in our study. First, it had a small sample size. As only an accurate sample size can make definitive conclusions about MRI parameters as prognostic markers in the resolution of SCG; the small sample size resulted in the predictors rarely meeting statistical significance.
Second, follow up duration was very short due to time constraints. It very important to have a long duration of follow-up as some patients may relapse after attaining remission. Similarly SCG lesions may show resolution after six months of follow up.
Third, we relied on self reported seizure frequency by patients for the study outcome. A proportion of patients may be unaware of their seizures, resulting in possible underreporting of seizures and overestimation of seizure remission as an outcome.
Fourth, contamination of the voxel from the surrounding oedematous brain parenchyma in MRS can be responsible for altered signal of the metabolites.
Conclusion
SCG is the most commonly encountered intracranial solitary granulomatous lesion. MRI is an excellent modality for making early and proper diagnosis of these lesions. Advanced MRI techniques as MRS and MT imaging are important in the prognostication of SCG. In our study a higher NAA/Cr and Cho/Cr ratio in the complete resolution group helped in predicting the resolution of the lesions and therefore we recommend a close attention to this important parameter, which can have impact on therapeutic decisions. However larger prospective studies with a longer duration of follow-up are needed to statistically establish the above observations.
