Introduction
Parkinson’s disease (PD) is a neuro degenerative brain disorder ensuing in a proven experimental (clinical and/or diagnostic) hypokinetic rigid syndrome. Apart from classical cardinal motor symptoms, the PD is accompanied by non-motoric symptoms. Amongst these are autonomic symptoms, for instance ortho static hypo tension, constipation plus urinary incontinence(dissoluteness) and they are testified by Parkinsonians and Parkinson disease patients and have been hinted as pre-clinical features or feature-manifestations (symptoms).1
However, the prime neuropathologic hallmark is the founding of cyto-plasmic inclusions termed as α-synuclein-enriched Lewy bodies as well as Lewy neurites. Suchα-synuclein accumulations are found in the entire brain, extremely stated in the substantia-nigra(SN).2, 3 The loss of ‘neural-cell’ is maximum copious and profuse within the SN_compacta. Remarkably, the pathological α-synuclein is also existing in the outer (peripheral) nervous-system, farther particularly in the vagal-nerve(VN), which plays significant role in autonomic control.4, 5 Researchers. 2, 3 and 5, 6 hypothesized that the neurotropic pathogen inflowing the brain might be tangled in the pathology of Parkinson disease. Which might follow via nasal/or gastric-route, triggering trans synaptic cell-to-cell, disease (protein, prion) alike, transmission-of α-synuclein. 5, 6
It is recommended that the pathogen occupies the head cerebral-brain through entering the nasal-mucosa, after wards, subsequently the ‘olfactory-bulb’ and the anterior olfactory nucleus (AON) into the olfactory anatomical-structures of the ‘temporal-lobe’ sequentially. The gastric-route starts in the enteric-nervous-system (ENS), where α-synuclein arrives the “Meissner’s and Auerbach’s plexus”. On or after this point α-synuclein is moved to the down chunk of the brain stem through the VN. α-synuclein blowouts as of the imprecise dorsal motor nucleus (DMN) into medulla plus rest-of the cerebral-brain. 6 Latest research in the primate animal model chains or defenses the ‘hypothesis’ that the proliferation of pathologic α-synuclein via VN causes the Parkinson disease. 7
Consistent (in-line) and in accordance with these outcomes, it was proved that the ‘vagotomy’ or ‘appendectomy’ immediate in life are connected by the lesser risk to progress Parkinson disease future/late in life. 8, 9, 10, 11, 12, 13, 14 The supplement (appendix) addendum postscript looks to be the greatest source-of α-synuclein, however the VN only appears to be the haulier,i.e., trasporter.11, 12, 13, 14, 15 According to these results/outcome, it has been presume that the cross-sectional-area(CSA) of the VN reduces in Parkinson`s. Certainly, experimental investigations were published and confirming the sizable reduction within the CSA of VN in Parkinson`s. 16, 17, 18, 19
It was hypothesized that atrophy-of VN previously follows at a pre-clinical-stage. 17 All these outcomes highly emphasize the possibility to apply the estimation of CSA of the VN-rostral to ‘carotid-bifurcation’ by employing ultrasound with good precision for the ultrasonography /ultrasonography (B-mode) as a steady as well as low-cost diagnostic test for the PD diagnosis. Furthermore, this strategy possibly detects people at with major health hard and at hazardous risk even preceding the experimental finding of Parkinson`s can be approved. Yet, it is notable to mention that two examinations by applying ultrasonograph at B - mode might not reinforce that CSA of VN was reduced in Parkinson`s.20, 21
The prime objective is to evaluate if the C S A is reduced in Parkinson`s or not. So, we hypothesize that the C S A of the VN is decreased in Parkinsonian patients contrasted to healthy-control population by applying the ultrasonograph. The secondary endpoint or objective is to measure if C S A correlates through the autonomic symptoms in Parkinson`s, as well as assessing the inter-rater consistency or dependability and assess distinctive dual techniques to quantify the C S A. To this end, we have done the cross - sectional study in which we gathered the C S A of the left and right VN by applying the ultrasonograph. The autonomic symptoms were measured through the consistent forms.
Materials and Methods
Ethical committee given the approval following the Helsinki principles and all participants given the written informed consent prior to their active participation. All the participants endured the ultrasonography ( B-mode) u l t r a s o u n d checkup of the VN plus complete within a short inquiry form/survey (questionnaires) regarding theautonomic grievances. The scales for Outcomes in Parkinson’s Disease-Autonomic Dysfunction; SCOPA-AUT.
Participants
Subjects selection criterion was based on the clinical diagnosis of Parkinson`s decided by neurologist/ neurophysiologist following the united kingdom (UK) Brain Bank criterion.22 Exclusion-criterion was if there are any neuropathology red-flag issues were found then the subjects were eliminated. The response was good with levodopa is considered. Fo comparison purposes healthy population without any abnormality is included. Exclusion criterion for two-cohorts were further neuro degenerative disorder plus aforementioned surgical-procedure within the vicinity of the vagal-nerve, for instance carotid endarterectomy or embedding the vagal-nerve stimulators. For the selection of the subjects both normal controls and patients sole neurologist. The normal-control population were selected at the clinic as of patients who were meant for ‘carotid-artery-ultrasound’.
Ultrasound precision sonograph
The sonograph of the vagal-nerve was accomplished by two qualified nerve-sonographers by applying the Philips-IU22 with a linear 17.5MHz transducer. The sonographer was not blinded to the participant’s identity, being a patient or a controller. The vagal-nerve was imagined in a coronal-plane (standard 2D axis) anterolateral to the common carotid artery (Figure 1). The vagal-nerve C S A was measured on spot throughout the examining period by sketching the inner-side of the hyper-echoic-epin-eural rim with the trace-function of the ultra sonograph system. At every-0side the C S A of the vagal nerve was measured twofold. The average-mean of the two measurements were applied for clinic-statistical inferences.
To ensure a good (smooth and efficient) inter rater dependability, the contour of the VN with in the hyper-echoic epi-neural-rim was defined by the two autonomous sonographer`s.
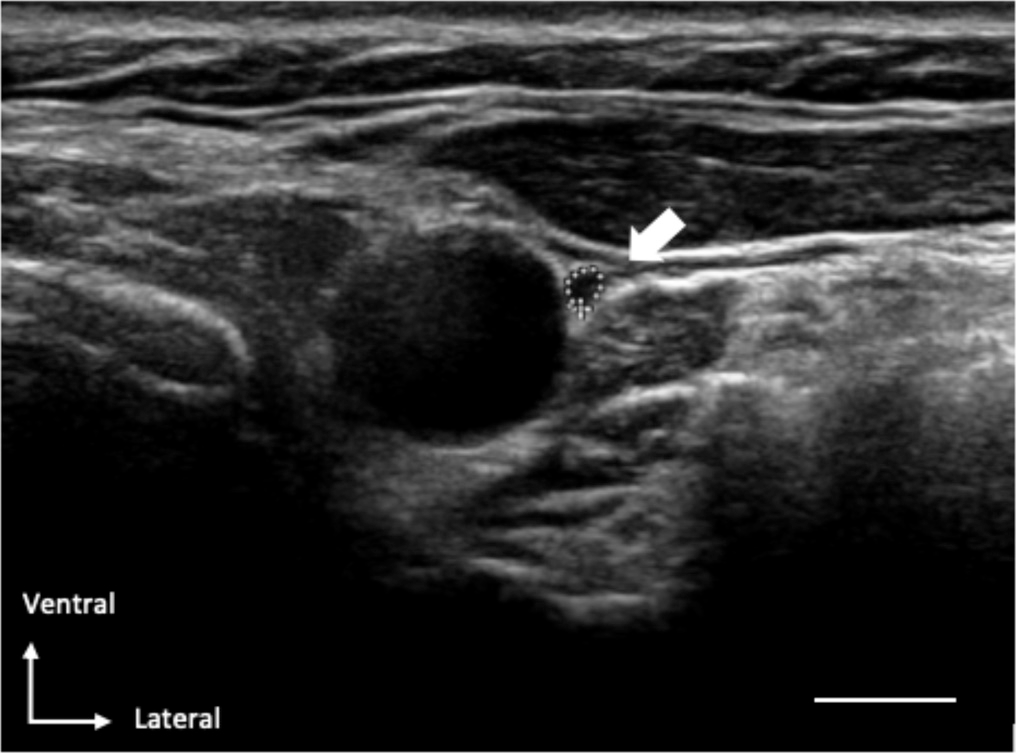
Figure 1
Characteristic illustration of an ultrasound-image in which the VN (with dotted-circle) is located ventro-lateral to the normal carotid-artery. The scale bar equals0.5cm.
To asses’ likely changes amid our strategy concerned as explained above, plus previous studies as well.,19 we measured the longest CS diameter ‘a’ plus diameter ‘b’ perpendicular to ‘a’ Walter,et.al.,1619 in 34 participants. The CSA was calculated using the following formula: (a*b*π)/4. The diameters were measured at the same place as the contour of the vagal-nerve was measured. This also happened immediately during the ultrasound.
Feedback form
On the same day as the ultrasound examination took place all participants filled in the SCOPA-AUT, a validated questionnaire about autonomic complaints. 23, 24 The SCOPA-AUT consists of six domains: gastrointestinal, urinary, cardiovascular, thermoregulatory, pupillomo- tor and sexual functioning. There are different numbers of questions for each domain, with a total of 23 questions. For each question a score from 0 to 3 points can be obtained, the maximum score that can be obtained is 69 points. Complete summation result employed for added analyses.
Clinico-statistical analysis
The baseline (electrical-baseline, i.e., zero line) properties as of the disease of Parkinson`s plus healthy- control population cohorts were balanced through independent-samples ‘t’-test for the age plus the outcome over S C O P A – A U T through the χ2 Chi-square test for the gender. All the subjects were measured twofold, left-side as well as right-side vagal-nerve. To account for the dependency amongst the two repetitive measurements/subject demoted/ marginalized-multi-level-model(MMM) analyses were accomplished, were done. To choose the best suitable covariance-matrix-structure (an unstructured matrix or a compound symmetry-matrix), the constricted highest probability ratio likelihood-test was employed. Initially, the MMM analysis-technique including the cohort (Parkinson`s and normal healthy control-populations), two-sides (left-right) plus their acquaintance was showed to calculate approximately the differentiation amongst/ concerning the two/twofold cohorts. Next, likely experimental plus demographics confounders were experimented sequentially in uni-variable model. Lastly, a compound M M M analysis-technique was done containing the or involving cohort, two - sides as well as all potential confounders. The ultimate model was constructed through the back ward removal of in-significant variables through concession of the cohort-variable.
The intra-class correlation-coefficient(ICC, (2,1) grouping as per the Shrout. 25 was computed in Mat Lab to comparability of the two various sorts of measurements for C S A as well as to relate the performances through the two autonomous sonographers. 25 The S P S S statistical tools-softwae was employed
Findings
Clinical-characteristics/Demographics
In total more than eight one
Males and females were evenly disturbed among groups. In the control group, 9(18%) participants had diabetes/mellitus devoid of difficulties, 31(62%) participants had cardio vascular-disease, plus 1(2%) had a poly neuropathy. The standard number-of-suppositories the participants consumed whilst ultrasound-test was 4(range:0-11). In a applicant the therapeutic record was unattainable. In the Parkinson cohort, 2(7%)patients had diabetes/mellitus devoid of problems, 5(16%)patients had cardio vascular-disease also patient had the record of poly neuropathy. The median number of prescriptions the applicants consumed despite their PD prescription at the time of ultrasound was 2 (range:0-14). mean totals coreon the SCOPA-AUTwas16±7 in Parkinsonians plus 13±8 points in the normal control population cohort. Yet, there was no considerable differentiation connecting the equal S C O P A – A U T score of 2 cohorts (p=0.078).
Table 1
Demographics, clinical scores and ultrasonograph of the Parkinson’s and control group.
The C S A of vagal-nerve
No change in the nerve echo-genicity plus no variants at the anatomical-structural level were monitored amongst the Parkinson`s cohort also control population. Firstly, we evaluated if a good inter-rater dependability was attained. The I C C demonstrated a solid promise among the neutral sonographers (I C C was≤0.969, p<.001, statistically highly significant with a 2 degree of freedom and 9.8Chi-square, Figure 2[A]). Then, we measured if our reconnaissance strategy was consistent by the previously explained approach to guess the C S A with thickness of the vagal-nerve in dualistic ways.26 The I C C demonstrated a vigorous yet promise amongst the two strategies to approximate the C S A (ICC=0.961, p<0.001, statistically significant). The average-mean C S A of the left-side VN within the Parkinson’s plus healthy population cohort was 2.10 and 0.57mm2 and 1.90 and 0.56mm2 plus right-side 2.54 and 0.70mm2, 2.24 and0.68mm2 (Figure 3). The M M M study exposed no major and meaningful relations amongst the two cohorts plus sides (,the t-test(80) 0.863,p<0.391) and was deleted as of model (TABLE II). Likewise, the M M M inferences were molded that the average-mean C S A of right-side vagal-nerve was substantially > left-side in Parkinson`s and in healthy-population groups, t-test(81),6.544, p<0.001,d0.379,95%CI [0.264,0.495] (right-left), Figure 2[B]. Values except gender are presented as means SD. For gender number and percentage of females are given. CSA, cross-sectional area in mm2. SCOPA-AUT, Scales for Outcomes in Parkinso ’ Disease - Autonomic Dysfunction. P-values are from comparisons between PD patients and the control group using an independent-samples t-tests for age, sex and SCOPA-AUT and a repeated measurement ANOVA for the CSA of the left and right vagal-nerve.
Correlation relating autonomic-symptoms, added variables, plus CSA of vagal-nerve
The uni—variable M M M inferences includes period(age), gender, cohort, diabetes/mellitus, cardio vascular disease or S C O P A – A U T as neutral variable demonstrated no meaningful impacts at a implication level of 0.05. Although the C S A deviation relating male - female was close to meaning (t (80) = - 1.867, =0.066, d -0.228, 95% CI [-0.470–0.015]), i.e., male - female.
The M M M investigation through the backward choice of important predictors, ensued within the model which is final-model containing the gender, plus sides as important-variables. The corrected contrast amongst Parkinsonians and P D patients plus healthy population was not important (t(78)= 1.786, p0.078, d=0.218, 95% C I [ -0.025, 0.461] (i.e., patients- healthy-population).
Figure 2
Scatter plots through our computation in Mat Lab statistical tools, demonstrating the linear correlation amongst [A] the C S A deterrence through dual autonomous sonographer`s, [B] the dissimilar strategies to seek the C S A perimeter (i.e.,circumference) over Y-axis plus thickness/dimeter a * b * π 4
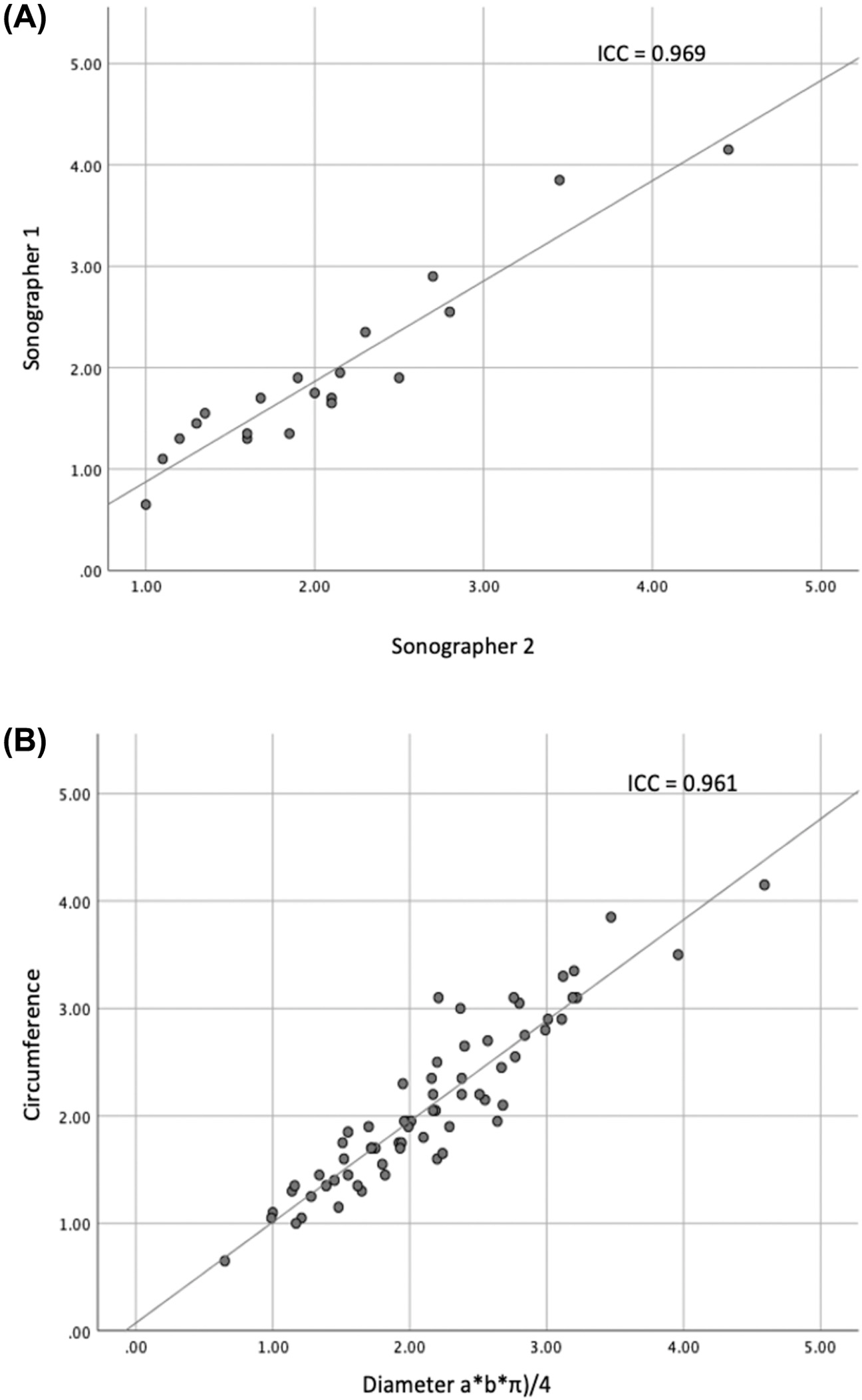
Figure 3
Barplots representing the mean of the CSA of the left-side and right-side vagal- nerve of the Parkinson’s disease and control group. The error -bars denote standard deviation (SD) of mean-average. The C S A, cross-sectional area is in mm2.
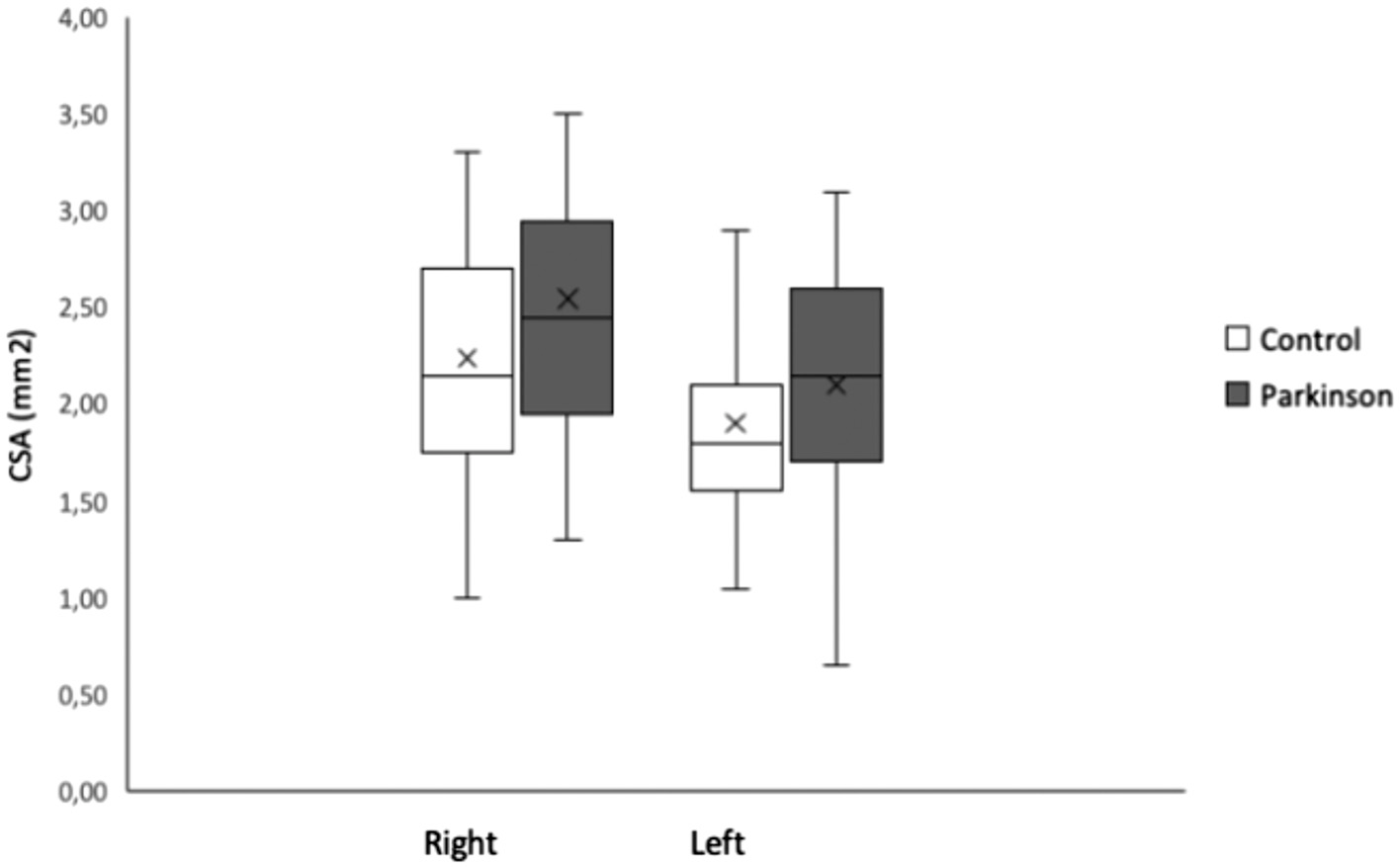
Table 2
Univariable and multi variable models.
Discussions and Thought Provoking
This study showed the C S A of the vagal-nerve is not distinctive in Parkinsonians and in Parkinson disease patients when assessed with normal-population group. Also, no link amongst C S A of vagal-nerve plus autonomic symptoms are evaluated by the S C O P A – A U T feedback as encountered. The C S A wasn`t altered by variables like age, gender/sex, diabetes/mellitus plus cardio vascular disease. Reliable by the previous studies when compared, the C S A of the right-side vagal-nerve was notably > in both the Parkinson`s and healthy-population. 16, 17, 18, 19, 20, 27, 28, 29
Our findings confirm the findings of the dual observations that were found no reduction in CSA of the vagal-nerve in PD patients. 20, 21 We were unable to replicate the reduction in CSA as reported by earlier studies. 17, 18, 19 Important to note is the high variation in CSA measured between studies. The mean CSA in control participants varies from 1.3 to 2.7 mm2 for the right and 1.1 to 2.6 for the left vagal-nerve, this is in line with recent published systematic review and meta-analysis. 27 In the PD groups the variation is even larger between studies. In our cohort we also found a high variance in CSA between individuals.
The validity of our findings is supported by several factors. First, we have shown a high inter-rater reliability. Second, our method (drawing a circle within the epineural rim to determine the CSA) has a high-correlation with previously used method, in which the longest cross- sectional diameter a and the diameter b perpendicular to a were used to calculate the CSA.19 Third, our findings replicate the well-known right-left difference in CSA is explained by the different functional anatomy of the vagal-nerve. The right vagal-nerve innervates parts of the small intestine, the colon and also a part of the gastric plexus. The left vagus nuclei terminate in the anterior plexus and from there on new branches go to the stomach, liver and the superior part of the abdomen. Fourth, the range of the CSA of the vagal-nerve we found is similar to previous reports. 17, 20, 27, 28
A possible reason why in our study no difference was found between PD and controls is the low prevalence of autonomic symptoms in our PD group. The mean score at the SCOPA-AUT for Parkinson patients is re-ported to be higher in some other experiments. 23 It could be proposed that subjects through additional autonomic symptoms might show a reduced CSA of the vagal-nerve. This could make the vagal nerve ultrasound a useful screening tool to detect autonomic dysfunction, but not to diagnose persons with PD in an early stage of disease or even in a pre- symptomatic phase. Conversely, our inferences didn’t give connection amongst the autonomic-features plus CSA of the vagal-nerve. Supplementary cohorts, yet, have seen a clear relation between autonomic symptoms and the CSA of the vagal nerve using the NMS- QUEST and assessment of heart rate variability. 17, 30, 26 There might be several reasons for the discrepancy. First, our PD group did score relatively low on the SCOPA-AUT. Next, the NMS-quest and use of heart rate variability might be better tools to assess autonomic dysfunction. It can be contended that the healthy population cohort wasn’t completely a healthy population.
Univariable models; where the ‘neutral-variables’ are associated by cross- sectional are of the left and right vagal-nerve. Multi variable models including all the independent values with significant value.
Conclusions
The CSA of the vagal-nerve can be measured with high accuracy. It is not reduced in PD patients and is neither correlated with autonomic symptoms, sex nor age. Therefore, we conclude that the results of this study do not support that a single ultrasound examination of the vagal-nerve is at this moment a suitable biomarker in the diagnosis of PD.
