Introduction
Our primary objective is to report and create awareness about Pelizaeus-Merzbacher disease, a dysmyelinating X-linked inherited disorder that can occur in females also and a possible reason can be an X-linked recessive inheritance pattern by gene mutation and skewed X inactivation.1, 2 The secondary objective is to underscore the significance of radiological diagnosis as a potential alternative to confirmatory genetic testing. Early diagnosis through MRI can help manage the patient in a better way by alleviating the anxiety of parents and working toward the best patient outcomes through supportive therapy. Pelizaeus-Merzbacher’s disease (PMD) is a rare X-linked inherited disorder caused by mutations in PLP1, the gene for protein lipoprotein (PLP), the major protein of CNS myelin. 3 PMD, is different from leukodystrophies like metachromatic leukodystrophy, adrenoleukodystrophy, and multiple sclerosis, as it is a dysmyelinating rather than a demyelinating disorder. In demyelinating disorders, myelin is formed, deposited around the axons, and then destroyed later. In dysmyelinating disorders such as PMD, normal myelination does not occur. 4 In this disease the body doesn’t produce enough myelin to protect the nerves thereby resulting in various neurological symptoms like impaired motor development with progressive spasticity, ataxia, choreoathetoid movements, dysarthria, and nystagmus. PMD affects males more than females and there are very few reported cases of females affected with PMD.
Case Presentation
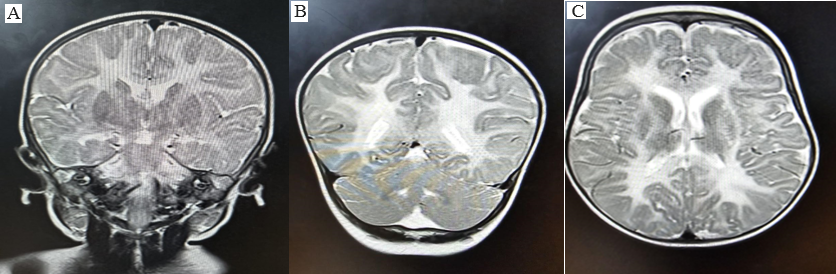
An 11-month-old female infant presented to the pediatric department with a history of regression of milestones over the last 2 months. According to the attendants, the infant has previously achieved milestones in all the domains of development appropriate for age up to 9 months. The infant lost the ability to stand without support and now only partial neck holding is present. The infant had previously achieved a pincer grasp but does not reach for objects now and shows no interest in toys. The general physical examination was normal. In the investigation, routine complete blood count (CBC), kidney function test (KFT), liver function test (LFT), and electrolytes were normal. MRI was done and T1WI, T2WI, FLAIR, DWI, and SWI sequences were exploited in axial, sagittal, and coronal planes. Bilaterally symmetrical altered signal intensity areas are seen involving subcortical & deep white matter of B/L cerebral hemispheres with involvement of subcortical U-fibres as well as internal & external capsule along with ventral brainstem showing T2/FLAIR hyperintense & T1 hypointense signal.(Figure 1) Genetic testing is not done due to financial and availability issues. On further evaluation, similar complaints of neuroregression were present in 2 other siblings, both females delivered at term (Ist delivery via Normal vaginal birth and the other via lower segment cervical section). In all 3 scenarios, there are no significant birth events and an uneventful antenatal period with no prior complaints. Both the elder siblings (female) are bedridden and completely dependent on other members of the family– The eldest sibling is 10 years, and the younger sibling is 9 years of age. Mother had a history of 2 spontaneous abortions at 2nd and 3rd month periods of gestation, for which no workup was done.
Discussion
Pelizaeus-Merzbacher disease (PMD) is an X-linked recessive disorder which occurs due to defects in the gene PLP1. Most females harboring heterozygous PLP1 abnormalities are asymptomatic. However, because of abnormal patterns of X-chromosome inactivation, some female carriers can be symptomatic.5 Our case sheds light on a rare case of PMD in a female infant. We propose that the patient's mother most likely has the affected X-linked gene which the patient obtained in an X-linked recessive inheritance with a skewed X inactivation. Also, our case report highlights the importance of performing brain imaging for early disease diagnosis.6, 7 Genetic testing is expensive and difficult in resource-limited settings. Our case was diagnosed with distinctive brain MRI findings seen in PMD only. Hence, radiological imaging of the brain is recommended as an alternative to genetic testing. There is no treatment available for the disease. The management of the disease involves a multidisciplinary approach taking care of various milestones through play/occupational therapy, hearing assessment followed by speech therapy, physiotherapy for contractures or spasticity, and behavioral therapy. The parents should be counseled for seizure control and antiepileptics if and as needed. There is current research going which incorporates stem cell therapy as a potent cure for the disease.8
Some important differential diagnoses of PMD including Salla disease, leukodystrophies, such as Metachromatic leukodystrophy, Adrenoleukodystrophy, Krabbe disease, Cockayne disease, and Canavan disease were ruled out based on characteristic MRI findings. However, Salla disease may manifest with nystagmus in the first months of life, as well as hypotonia and cognitive impairment. MRI reveals arrested or delayed myelination.9 MRI scans in adrenoleukodystrophy usually reveal a disease-based regional predilection for associated abnormalities (eg, occipital white matter in adrenoleukodystrophy, frontal white matter in metachromatic leukodystrophy).10 Kinoshita M et al reported a three-year-old female patient with a chief complaint of developmental delay. Apart from poor eye contact, the physical examination was normal. Since no chromosomal evaluation was performed, a chromosomal microarray testing was performed. A review of the geneticist report indicated that the patient carries a deletion of at least 2.26 Mb within the cytogenetic band Xq22.1 to Xq22.2 which is known to contain 39 genes. Out of the 39 genes, proteolipid protein 1 is associated with a known clinical disorder- Pelizaeus Merzbacher Disease.11 Gagan et al reported that even a female child with microcephaly, neurodevelopmental delay, neurodevelopmental regression, ataxia, and decreased scholastic performance can be considered as PMD and subsequently confirmed by MRI that showed generalized demyelination along with atrophy of cerebrum and cerebellum. However, it is best confirmed by genetic study. 12 One limitation of our case report is the nonavailability of genetic testing at our hospital.
Conclusion
This case report presents a rare instance of PMD in an 11-month-old female, underscoring the necessity of early radiological diagnosis through MRI in resource-limited settings where genetic testing is not feasible. Distinctive MRI findings in PMD can facilitate timely diagnosis, enabling better patient management and alleviating parental anxiety. This case emphasizes the importance of considering PMD in differential diagnoses of neurodevelopmental regression even in females and advocates for the role of MRI as a vital diagnostic tool in identifying PMD, especially when genetic testing is unavailable.
Author Contributions
We take full responsibility for my substantial contributions to this work. This includes involvement in the conception and design of the study, acquisition and analysis of data, interpretation of results, and drafting of the manuscript. Additionally, we have been actively engaged in substantively revising the work to ensure its accuracy and integrity. Our dedication and efforts have played a pivotal role in shaping the outcome of this project.