Introduction
Psychogenic nonepileptic seizures (PNES) are paroxysmal changes in motor, sensory, autonomic, or cognitive functions, mimicking true seizures, however without electrical corelate.1 Also known as functional seizures (FS), dissociative seizures, non-epileptic seizures, or psychogenic seizures, PNES are involuntary behavioural reactions to internal or external stimuli. It is a ubiquitous subtype of functional neurological disorder (FND), tabulated under the somatic symptom category and related disorders in DSM-5. Functional disorders are commonly precipitated or worsened by stressors. Patients with FND are associated with comorbid psychiatric illness and are at a higher risk of multiple somatic symptoms and worsening of these under stressful circumstances.2
COVID-19 pandemic and lockdown had significantly impacted the population's mental health worldwide especially in individuals with pre-existing psychiatric conditions.3 Subjects with FND can show either stability during the COVID-19 pandemic or a worsening frequency and intensity of functional episodes.3 COVID-19 pandemic has had a profound and severe psychological impact in subjects with PNES.4
Being at a tertiary care centre, our initial observations showed an increase in occurrence of PNES during the pandemic. Hence this study was targeted to assess the possibility of increase in PNES during the pandemic and the factors associated with this worsening in a cohort of subjects with PNES diagnosed at a tertiary hospital.
Objective
To identify the frequency of PNES during corona virus pandemic for a period of 6 months from March 2020 to August 2020 and compare it with a 6-month pre-pandemic period from March 2019 to August 2019 and identify possible etiology causing aggravation of PNES.
Materials and Methods
Subjects
This was done in a tertiary referral centre in South India, which caters to referred neurology patients from elsewhere. In this cross-sectional comparison study, which was executed during the COVID outbreak, adult subjects visiting the neurology outpatient/ inpatient department during a 6-month period at the peak of the pandemic, with PNES documented by short- or long-term video-electroencephalography (video-EEG) or home video-recording were included and compared against a baseline 6-month period (of the previous year) in the pre-pandemic period. The video-EEG/home video recordings were reviewed by experts in epilepsy to determine if the features were suggestive of PNES. After systematically screening all the video recordings, cases of PNES were identified by the following criteria: Patients who had
Home video recordings, after review by an expert in epilepsy, favouring PNES (at least 2 of the common features of PNES as given in Table 1)
Video-EEG proven PNES (events having no electrographic corelate)
Patients having functional neurological disorders not mimicking seizures (eg: functional movement disorders, weakness) were excluded by systematic analysis of videos. 5
Electroencephalography (EEG)
Video-EEG was done on a 16-channel digital EEG acquisition system (NicVue, Nicolet-Viking, USA); scalp electrodes were pasted as per the International 10–20 system. Outpatient video-EEG was captured for forty minutes (twenty minutes of awake and twenty minutes of sleep record). Hyperventilation for three minutes and photic stimulation in wakefulness were part of the standard recording. 6 Suggestions were given as part of the protocol for PNES recording and any patient with doubtful home video semiology, underwent video-EEG till event capture.
Patient information
The Institutional Ethics committee approved the study. The elements that composed the study are detailed below.
PNES: Data regarding the type/pattern, phenomenology, and frequency of PNES.
Demographics, clinical data, felt (perceived)stress, access to health care and level of social isolation (no/ partial / total isolation).
Presence of psychiatric comorbidity was assessed. Anxiety and depression were scored by Hospital anxiety and depression scale (HADS-A for anxiety and HADS-D for depression) and sleep quality were recorded by Pittsburgh Sleep Quality Index (PSQI) questionnaire. The HADS questionnaire has seven items each for depression and anxiety subscales. Scoring for each item ranges from 0-3, with three denoting highest anxiety or depression level. A total subscale score of >8 points to mild or borderline symptoms of anxiety or depression while > 11 denotes clinically significant anxiety or depression. The PSQI questions are rated under 7 domains each scored 0-3, with 3 denoting severe difficulty. The scores range from 0 to 21 and a global score >5 is considered as a significant sleep disturbance.
Results
26/1350 patients (65% female; age-26.7 years [range=4-68]) seen in neurology outpatient/inpatient department during the pandemic (6 months) were diagnosed to have PNES as compared to 49/ 7125 (71% female; age 28.9 years [range= 5-63]) seen in the pre-pandemic period showing 2.83 times increase. 76.9% (20) had an abnormal score (>11) on HADS-A or HADS-D. 46% (12) had anxiety, 11.5% (3) had depression while 19.2% (5) had coexistent depression with anxiety.80.77% (21) had a poor sleep quality with a PSQI score of > 5. There was strong correlation between higher anxiety scores (p < 0.001) and poor sleep quality (p< 0.005) with PNES frequency.
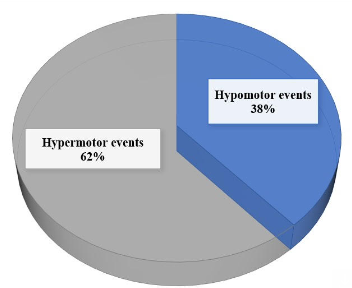
Subjects with PNES were significantly more likely to report that stress and poor sleep were their typical event precipitants [53.8% vs 32.6%, p = 0.024, and 46.1% vs. 12.2%, p < 0.001, respectively] (table 2). Headache of any character or migraine was reported in 50% of the patients as compared 24.5% in the pre-pandemic period (p=0.04). 61.5% patients were unable to get a specialist/neurologist appointment for their symptoms during the the initial 4 weeks of the pandemic. 8% of patients had the onset of PNES within 3 weeks of COVID-19 infection while no clear triggers could be identified in 19% of patients. The phenomenology of the PNES was hypermotor in 62% of patients while it was hypomotor in 38% of patients (Figure 1).19.2% of the patients with PNES had coexistent epilepsy while 7.7% of them had a previous history of PNES.
Table 1
Distinguishing clinical features between PNES and epilepsy
Table 2
Comparison of demographics, triggers, and comorbidities in patients with PNES the COVID-19 pandemic vs baseline pre-pandemic period
Discussion
The estimated incidence of PNES is 3.1 per 100,000 per year, and the prevalence is 108.5 per 100,000. The highest prevalence is among 15- to 19-year-olds at 59.5 per 100,000. However, epilepsy in comparison has a higher incidence of 62 per 100,000 per year and a prevalence of 1,200 per 100,000. 7 PNES can demonstrate stereotypy and the presence of psychogenic seizures do not exclude a concurrent diagnosis of epilepsy, since the two often coexist in 10% to 30% of patients. Certain behaviours, such as side-to side turning of the head, clonic jerking of all four extremities without loss of consciousness, compulsive eyelid screwing and pelvic thrusting are more commonly associated with psychogenic seizures (Table 1). The distinction is occasionally difficult on clinical grounds alone, since the behavioural manifestations of frontal lobe seizures can be extremely unusual, and in both cases the routine surface EEG maybe normal.
COVID-19 pandemic has caused an overall increase in post-traumatic stress symptoms and depression. Patients with preexisting psychiatric disorders have reported worsening of symptoms. A decrease in psychological well-being was observed in the public. 8 However, studies specifically addressing the pandemic’s impact on PNES is scare.
Our cross-sectional comparison study was designed to determine the incidence of PNES during the pandemic which showed 2.83 times increase in its occurrence in comparison to the pre-pandemic period which was taken as the baseline. During the COVID outbreak, psychological stressors due to restrictions in movement and social interactions may have generated higher rates of PNES onset or recurrence. Severe social barriers are akin to imprisonment and can affect the mental health of the confined. Patients with preexisting PNES were more likely to relapse. Increased stress as well as restrictions to avail health care played significant roles in their deterioration.
76.9% of the PNES patients had anxiety or depression as demonstrated by the HADS-A and HADS-D scores respectively. 46% had anxiety, 11.5% had depression while 19.2% had coexistent depression with anxiety. Overall, there had been a 25% increase in the prevalence of anxiety and depression globally during the pandemic as reported by World Health Organisation (WHO). Females and younger adults experienced more anxiety.8 This could explain the higher incidence of PNES during the pandemic especially in patients who had greater anxiety or depression. Use of anxiety and depression scales has allowed us to further determine the impact of mood symptoms on PNES control.
80.77% of the PNES patients had a poor sleep quality as demonstrated by the high PSQI score. While several studies in subjects with corona virus infection have shown poor sleep quality and increased incidence of insomnia during the active phase of infection and convalescence, it is noteworthy that the control group (patients without COVID-19 infection) in the study by Al-Ameri et al, showed 78% having poor sleep quality. 9, 10 This could be attributed to associated mood disturbances, disrupted life routine, unexpected socioeconomic consequences or even an asymptomatic infection in few. Recognizing and treating sleep disorder is important especially with coexistent stress or mood dysfunction.
Our study showed that 8% of patients had PNES within 3 weeks of COVID-19 infection. New onset PNES following COVID-19 vaccine has been reported which has been labelled as immunization stress-related response (ISRR). 11 Precipitation of FNDs including PNES and its overwhelming coverage in media (social and print) have had a negative impact on vaccination campaigns. 12 Severe infection can cause cortical hyperexcitability through metabolic disturbances, hyperpyrexia or direct viremia. New onset acute symptomatic seizures and status epilepticus have been described with COVID-19.13 potentially suggesting both neurotropism as well as an immune mediated aetiology.14 True seizure aggravation can also, therefore, be a pandemic induced outcome.
19.2% of the patients with PNES had coexistent epilepsy making it imperative for a direct contact with a neurologist for characterisation of the event and accurate diagnosis. As the management of PNES and breakthrough seizure would be disparate, a positive diagnosis of PNES would focus on counselling and behavioural therapy while preventing the inadvertent increase in antiepileptic medications.
Literature evidence shows that some subjects with PNES have had a relative improvement in psychogenic seizures in the early part of first two peak phases of COVID-19 pandemic which is contrary to our findings.15 This could be attributed to reduction in their exposure to stressful settings at work and more comfortable environment at home. These PNES patients had capitalised on better sleep and other healthy lifestyle habits related to the closure of schools and workplaces and their improved status maybe attributed to additional sleep and isolation from work stress. For some patients with social anxiety, isolation leads to reduced stress levels. 16 Restrictions imposed by the lockdown may decrease their perceived work or academic burden, causing a reduction in PNES.
Our study has two highlights first, there was 2.83 times increase in PNES during the pandemic. Second, higher levels of stress, anxiety, depressive symptoms, and poor sleep quality were more in this PNES cohort of which higher anxiety scores were the strongest predictor of increased PNES frequency. The robustness of the study lies in the fact that confirmatory video evidence (either home or video-EEG) was obtained and corroborated independently by 2 experts for PNES and a criterion (Table 1) was used in its confirmation.
The limitations of this study, include small sample size and absence of follow-up data. Incidence of other functional symptoms (including nonspecific headache, somatoform pain etc) could be altered leading to their reduction, and a relative increase in functional motor manifestations. Restrictions to public travel were enforced during this period, especially on air and rail travel and only subjects who had private vehicles of their own or were able to hire a cab / driver were able to present themselves. So we believe that our numbers could be an understatement and true number of PNES would have been more. The strength of this study is that though there are many papers on COVID-19 and epilepsy very limited data is available on its impact on PNES and our article addresses this vacuum.
Conclusions
We found the increased occurrence of PNES during the viral outbreak to be striking which was 2.83 times high as this cohort is vulnerable during stressors. Presence of mood disorders like anxiety and depression, increases the risk of PNES aggravation. COVID -19 as a pandemic situation was gruelling in itself plus the increased occurrence of PNES and the associated additional hospital visits and financial burden makes it a 'double whammy'.