- Visibility 66 Views
- Downloads 10 Downloads
- DOI 10.18231/j.ijn.2024.006
-
CrossMark
- Citation
Investigation of anticancer properties of cinnamon phytochemicals on protein expression in glioblastoma multiforme cell lines (U87-MG)
- Author Details:
-
Tina Mary George
-
Prabha M *
-
Mukta S Patil
-
Soumya Sakshi
-
Shifa
Introduction
Glioblastoma Multiforme (GBM) is one of the most aggressive types of brain cancer, having a high incidence and fatality rate.[1] Tumours in the eloquent cortex, brain stem, or basal ganglia are not surgically treatable, and patients with these tumours typically have a poor prognosis. [2] The median survival time for GBM patients was roughly 15 months. [3] Despite the current multimodal standard of care, which includes surgical resection as well as adjuvant chemotherapy and radiation, the prognosis is still dismal. [4] Providing an adjuvant to chemotherapy has been the focus of several research over the years in order to improve response rate to chemotherapy, reduce side effects, and minimise treatment resistance. It goes without saying, minimizing side effects and improving quality of life are critical for cancer patients. [5] Although conventional drugs, like Temozolomide (TMZ), the only standard chemotherapy for patients with GBM, may result in a minor increase in median survival, they may only be palliative care for the majority of patients whose tumours cannot be completely treated. Developing efficient chemotherapy that can traverse the blood-brain barrier and selectively destroy cancer cells while not hurting normal cells, on the other hand, remains challenge and a huge issue. Prior therapeutic radiation, lower susceptibility to allergies, and altered immunological response are all risk factors for GBM. [6] As novel medicines are sorely required, naturally occurring chemical compounds have been studied. [7] Natural products are appealing as anticancer agents because of their low toxicity profiles. This allows them to be used at high doses safely or added to existing regimens. Plants, which are rich in bioactive chemicals, have the potential to be attractive alternatives or adjuvants for cancer treatment. Several medicinal plants showed anticancer potential without creating bothersome side effects, making these safe chemicals preferred for cancer therapy.
Cinnamon is a naturally occurring component with a variety of pharmacological properties, including anti-oxidant, anti-microbial, and anti-cancer properties for cancer cell apoptosis.
Apoptosis dysfunction is important in the genesis and development of cancer. [8] A considerable quantity of sodium benzoate generated by cinnamon crosses the blood brain barrier rather efficiently, allowing access to the brain. [9] We worked with Cinnamomum verum in our project. The bark and leaves of C. verum contain significant quantities of eugenol (90.2%) and cinnamaldehyde (44.2%). [10] Fresh dry cinnamon bark was extracted using methanol as a solvent in a Soxhlet extractor, followed by a Rotary vacuum evaporator, for qualitative analysis. Phytochemical screening techniques using various chemical reagents identified the presence or absence of several phytoconstituents such as carbohydrate, glycoside, protein, tannins, saponins, flavonoids, and terpenoids. The presence of these chemical elements in the extract suggests that if adequately screened, the plant might provide pharmaceutically significant medications. [11], [12]
Cell culture is an important tool in cancer research because it allows scientists to investigate the biology of tumor cells in a controlled environment. [13] Furthermore, because of the simplicity with which cell culture can be scaled up and the availability of multi-channel liquid handlers, it has become a cost-effective platform for high-throughput drug screening. However, rigorous cell culture methodology is essential for research reproducibility among laboratories throughout the world, as well as for translational potential from bench research into clinical settings. [14] U-87 brain tumor cell line was cultivated for further study in our project. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) technique was used to determine the best concentration and time for treating cells with Cinnamon aqueous extract. Cells were sown in 96 well plates. Light absorption at 570 nm was measured using a plate reader to test cell viability. [15] The most widely used method for producing high resolution analytical separation of protein mixtures is Sodium Dodecyl Sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The method starts with denaturing component proteins with an anionic detergent that also binds to them, imparting a negative charge corresponding to their molecular mass to all proteins. Electrophoresis over a porous acrylamide gel matrix is then used to separate proteins with high precision. [16] Proteins were therefore isolated by electrophoresis on SDS-polyacrylamide gel. The bands of the individual wells were studied and compared to the marker. Furthermore, the phytochemical extract tested on glioblastoma U87-MG cell lines to determine cancer cell survival and protein expression in order to uncover anticancer characteristics. With this context in mind, the current effort intends to extract phytochemicals from cinnamon as a cure for GBM.
Materials and Methods
Procuring of cell lines and other materials
U-87 MG cell lines was purchased from the National Centre for Cell Science (NCCS) in Pune, India which was maintained in Dulbecco’s minimum essential medium (DMEM) in a T25 cell culture flask, Cinnamon verum was brought from an online site (JioMart), analytical grade methanol was brought from Vasa Scientific, Bengaluru.
Cinnamon extraction from cinnamon verum
Hot extraction
35g of finely crushed cinnamon was weighed, placed in the thimble of the Soxhlet apparatus. The extraction chamber was filled with 250ml of solvent (methanol). Heating mantle was set at 400C. The apparatus was left running for 6-7 siphoning cycles. The extracted liquid was filtered using Whattman filter paper and placed in a sterile container This extract was later separated from the solvent with the help of the Rotary vacuum evaporator. The solvent was evaporated until 250ml of it was removed. The extract was then added to a petri dish and dried by placing the plate on water bath at 500C. [17]
Cold extraction
10g of finely crushed cinnamon was weighed and put into a conical flask with 50ml of methanol. This was kept in room temperature for 1 week. After a week, phytochemical tests were performed.
Phytochemical analysis of cinnamon extract
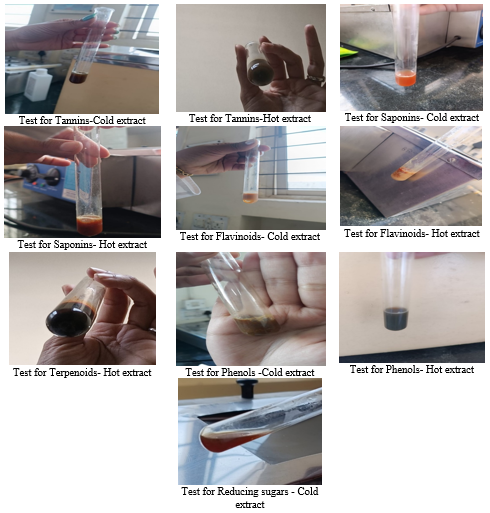
Phytochemical analysis was performed on the aqueous extract to detect different contents using Sofowara, Trease, Evans, and Harbone standard procedures.[18] In the test for alkaloids, the aqueous cinnamon extract was treated with Mayer's and Wagner's reagents. Tannins were determined by mixing the aqueous cinnamon extract with a few drops of ferric chloride solution. Saponins were tested by shaking the aqueous cinnamon extract briskly with distilled water in a test tube and warming it. To test for phlobatannins, the aqueous cinnamon extract was mixed with 1% HCl and boiled. Flavonoids were determined by combining the aqueous cinnamon extract with 10% lead acetate. In terpenoids test, the aqueous cinnamon extract was diluted in chloroform and dried. In the Liebermann's Glycosides test, aqueous cinnamon extract was diluted in chloroform and acetic acid, chilled in ice. Steroids were tested by aqueous cinnamon extract dissolved in chloroform and strong sulfuric acid. Phenols were measured by adding ferric chloride solution to the aqueous cinnamon extract. [19] Drops of Fehling's solution was added to aqueous cinnamon extract and boiled to test for sugar reduction. [20]
Maintenance of cell lines
Cell lines were grown in sterile flasks at 370C in DMEM supplemented with 10% Fetal Bovine Serum (FBS) and kept in a 5% CO2 incubator until they reached 90% confluency. For three days, 20-30 ml of fresh DMEM with 10% FCS was introduced and seen under an inverted microscope.
Sub Culturing
The spent media was stored aseptically for further analysis. To avoid disrupting the connected cell layer, the wash solution (DPBS) was added. The petri dish was filled with the pre-thawed dissociation reagent, Trypsin-EDTA (3–4 ml) after removing DPBS. The cells were examined for detachment using an inverted microscope. The cells were placed on several petri dishes containing the growth medium for 90% confluency. The plates were incubated at 370C, 5% CO2 in the CO2 incubator. They were allowed to grow for 24-48 hours.
Cryopreservation
The U87-MG cells were preserved at a very low temperature (-1960C) in order to maintain the genetic stability of biological material and metabolic inertness. The cells are preserved with the addition of cryoprotectants such as dimethyl sulphoxide which minimizes the ice crystal formation. An inverted microscope was used to inspect the cells for 90% confluency and to confirm the lack of bacterial and fungal contamination.
Determination of Cell Viability by MTT Assay
This colorimetric assay is used as a measure cellular metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity. [21] The procedure is carried for a period of three days.
Day 1- Cell seeding: The cells were collected from previously maintained culture plates. 100 µl of 4-5 ml of FBS and DMEM was added into each well of 96 well plate. The plate was observed under the microscope and incubated for 24 hours.
Day 2 -Addition of extract: A stock solution was made by dissolving the dried cinnamon extract (1.310 g) into 4ml methanol. This solution was filtered using a syringe and poured into 15ml tube labelled as Cinnamon extract sterile. 6 vials were taken and labelled as 1,2,3,4,5 and 6. Serial dilution of the cinnamon extract to be added were prepared as follows:
|
Vial Number |
Concentration of cinnamon extract (mg/ 100 µl) |
|
1. |
1.650 |
|
2. |
0.825 |
|
3. |
0.412 |
|
4. |
0.206 |
|
5. |
0.103 |
|
6. |
0.51 |
|
7. |
0 |
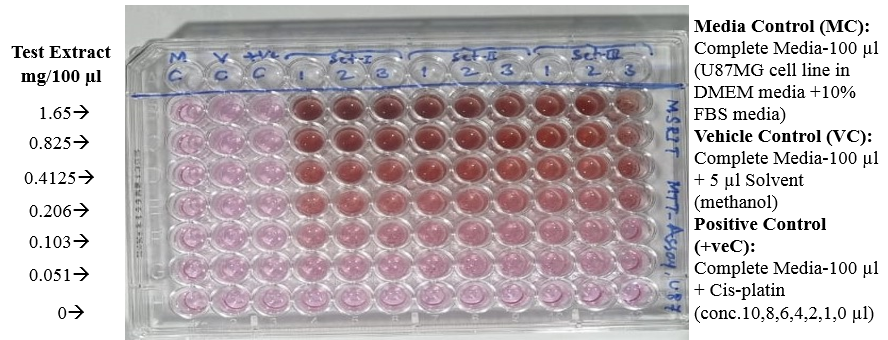
Day 3- Adding MTT and reading plate:
100µl of U87MG cells+ FBS was added row-wise, into the wells. 10 µl of MTT dye was added and incubated for 4 hours. Purple colored formazan crystals were formed upon reaction of U87MG cells with MTT.
Dead cells did not produce these crystals and thus, they could be differentiated. After incubation for 4 hours, DMSO was added after removing the previous solution and again incubated for 10 minutes. Reading of all the wells was done using ELISA reader at 570nm.
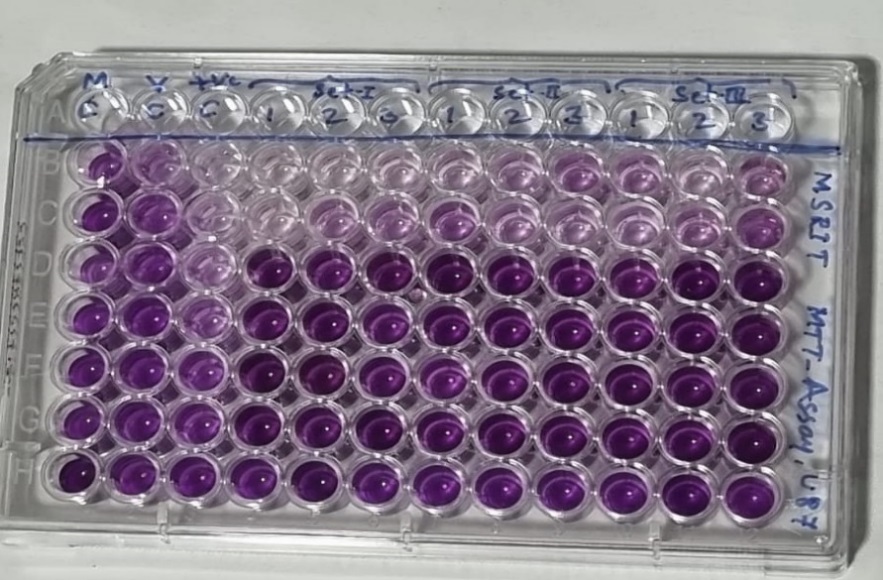
The MTT assay was used to assess the half maximum inhibitory concentration (IC50) of the medication (Cinnamon extract) in the U87-MG cell line following treatment. A positive control of 100% lysed cells was used to calculate the proportion of dead cells. Before adding the MTT, the cells were lysed by freeze-thawing and pipetting. As a blank value, the absorbance readings for the positive control (100% lysed cells) are averaged. The absorbance values at 570 nm versus cytotoxic agent concentration were plotted, and the IC50 value was calculated by locating the abscissa value corresponding to one-half of the maximum absorbance value (IC50 is the inhibitory concentration of cytotoxic agent required to kill one-half of the cell population) .
Extraction and Separation of Protein by SDS PAGE
When positioned in an electric field, a charged molecule migrates to the electrode with the opposite sign, according to the principle of SDS page. The relative mobility of charged species influences the separation of charged molecules.
Cell Culturing for SDS-Page: Cells were cultured in 3 different petri plates labelled as Control, 0.6mg/100 µl and 0.8mg/100 µl for sds page in which 0mg/100 µl, 0.6mg/100 µl and 0.8mg/100 µl extract (sample) was added respectively. Media from these plates containing extract and cells was collected and added to 3 centrifuge tubes and centrifuged for 10 minutes at 1500 rpm. The resulting supernatant was discarded and pellet was collected.
5 vials were taken and 10 µl of dye was added to each vial. 25 µl of protein ladder was added to 1st vial, 40 µl of control (untreated) was added to 2nd vial. 40 µl of cells treated with 0.6mg/100 µl extract was added to 3rd vial. 40 µl of cells treated with 0.8mg/100 µl extract was added to 4th vial. 50 µl of cells treated with 0.8mg/100 µl extract was added to 5th vial. These vial samples were put into the wells. The gel was transferred to a tray containing water and washed for 5 minutes. 20ml of Ezee blue was added to stain the gel, overnight. The gel was de-stained with water and placed on a white paper. The distance moved by each marker and the samples were noted down. [22]
Protein Estimation by Lowry’s Method
The quantity of protein in the cinnamon extract treated U87-MG cells (0.6 and 0.8 mg/100 µl) and control (cinnamon extract untreated U87-MG cells) was estimated by Lowry’s method. A graph was plotted with concentration of protein versus optical densities (OD) at 660nm.
Results and Discussions
Extracting cinnamon from cinnamon verum
Cinnamon verum was extracted using the Soxhlet equipment, and the solvent from the extract was evaporated using a Rotary vacuum evaporator. The extract was dried by air and then dissolved in methanol. After completely vaporizing the methanol, 1.310g of extract was obtained.

|
Phytochemicals tested |
Cinnamon Extract |
|
|
Hot Extract |
Cold extract |
|
|
Alkaloids |
+ |
+ |
|
Tannins |
+ |
- |
|
Saponins |
+ |
+ |
|
Phlobatannins |
- |
- |
|
Flavonoids |
+ |
+ |
|
Terpenoids |
+ |
- |
|
Glycosides |
- |
- |
|
Steroids |
+ |
- |
|
Phenols |
+ |
+ |
|
Reducing sugar |
- |
+ |
|
MTT Wells |
Average OD at 570nm |
Cell Viability (in %) |
Cell Death (in %) |
|
Media Control |
1.264 |
100 |
0 |
|
vehicle control |
0.668 |
52.858 |
47.142 |
|
Positive control (µl) |
|||
|
10 |
0.076 |
6.013 |
93.987 |
|
8 |
0.157 |
12.421 |
87.579 |
|
6 |
0.243 |
19.225 |
80.775 |
|
4 |
0.391 |
30.933 |
69.066 |
|
2 |
0.692 |
54.746 |
45.253 |
|
1 |
0.827 |
65.427 |
34.573 |
|
0 |
1.264 |
100 |
0 |
|
Test extract (mg/100 µl) |
|||
|
0 |
1.264 |
100 |
0 |
|
0.051 |
1.435 |
113.555 |
-13.555 |
|
0.103 |
1.287 |
101.846 |
-1.846 |
|
0.206 |
1.228 |
97.117 |
2.883 |
|
0.4125 |
1.279 |
101.178 |
-1.178 |
|
0.825 |
0.3 |
23.717 |
76.283 |
|
1.65 |
0.211 |
16.684 |
83.316 |
Phytochemical analysis of cinnamon extract
The aqueous extract prepared with the concentration of 2 mg/2ml in methanol was used to determine the constituents present in the extract.
The results of phytochemical analysis are shown in [Table 2]. The aqueous extract isolated was found to be positive for Alkaloids, Tannins, Saponins, Flavonoids, Terpenoids (only for Hot extract), Phenols and Reducing sugars (only for Cold extract) and negative for Phlobatannins and Glycosides. Corresponding observations of the tests performed is given in Figure 4 which have shown positive results for the phytochemicals mentioned in [Table 2]. The positive phytochemical results have shown to play an important role in inhibiting cancer properties.
Determination of U87-MG Cell viability by MTT assay
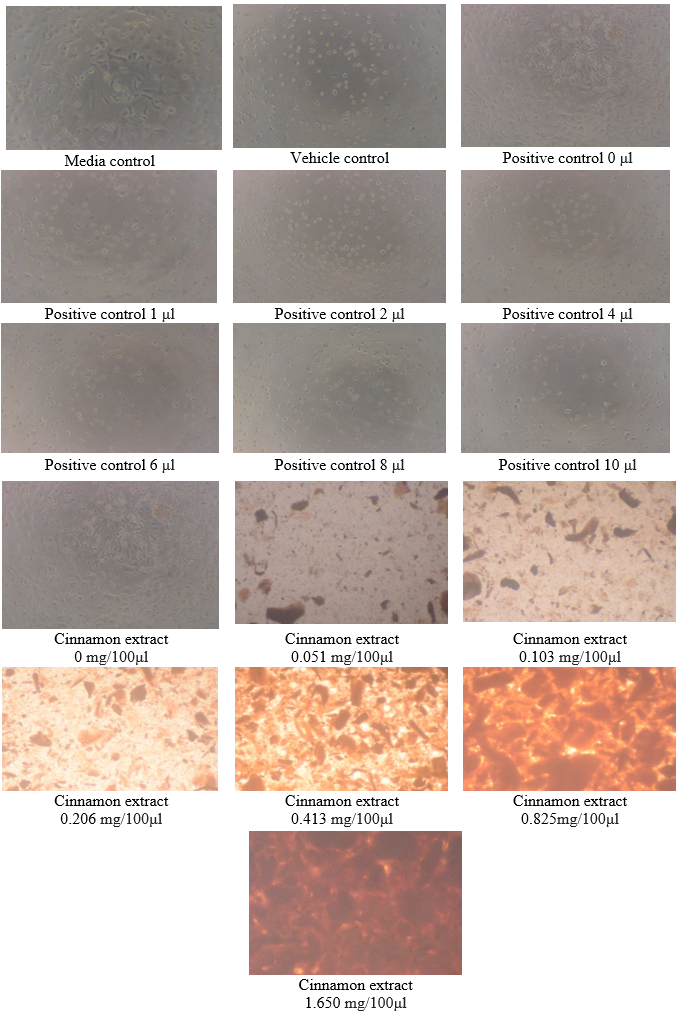
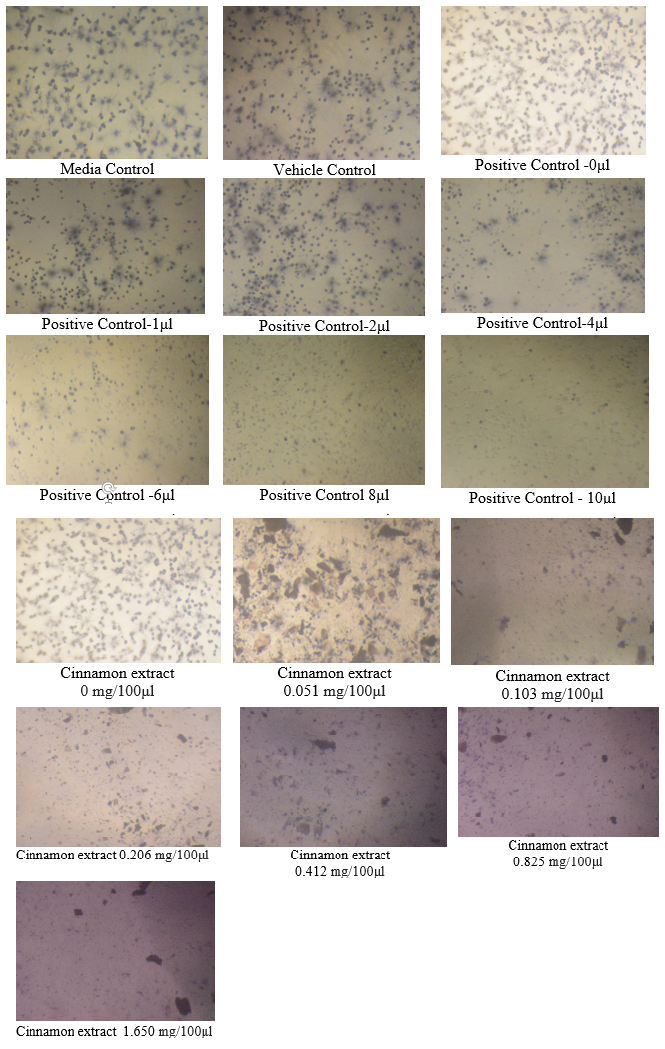
The MTT test was performed to determine the half maximal inhibitory concentration (IC50) of cinnamon extract after treatment with U87-MG cell line. The behavior of U87-MG cells after exposure to cinnamon extract and after the addition of MTT is shown in Figure 5 and 6.
As per this test, the cell viability of cinnamon extract with concentration of 0 mg/100μl (Control- untreated U87-MG cells with cinnamon extract) was 100% whereas the cell death was 0%.
The cell viability at cinnamon extract concentration of 0.051 mg/100μl is shown to be highest and cell death at maximum cinnamon extract concentration of 1.65 mg/100μl is highest. Thus, MTT assay depicted that higher concentrations of cinnamon extract is found to be toxic when tested on U87-MG cell line.([Figure 7], [Figure 8]) The IC50 value of the Cinnamon extract was found to be 0.729. ([Figure 9])



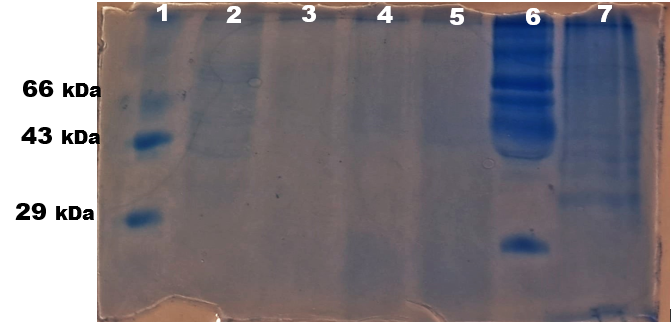
Extraction and Separation of Protein by SDS PAGE
The samples were subjected to SDS PAGE and the bands ([Figure 10]) were observed. The protein bands observed in SDS PAGE showed high intense band for lane 2: control (untreated) as the U87-MG cells were alive, the protein content was more. Among the treated samples, lane 3: 40 µl of U87-MG cells treated with 0.6mg/100 µl cinnamon extract showed very light band, indicating the decrease in U87-MG cell viability whereas lane 4 and 5 showed comparatively brighter bands indicating U87-MG cell viability higher than in lane 3.
Protein Estimation by Lowry’s Method
The absorbance value of Control (C) gave a protein concentration of 48µg, 0.6 mg/100µl gave a protein concentration of 24µg and 0.8 mg/100µl gave 40 µg.
Thus, protein expression significantly reduced in 0.6 mg/100µl cinnamon phytochemicals treated U87-MG cells and comparatively there was a slight increase in higher concentration but, lower compared to the control GBM cells
Conclusions
The Cinnamon extract phytochemicals such as Alkaloids, Tannins, Saponins, Flavonoids, Terpenoids (only for Hot extract), Phenols and Reducing sugars (only for Cold extract) showed significant anticancer properties for cell viability which reduced in cell number by showing IC50 0.729. The cell viability at cinnamon extract concentration of 0.051 mg/100μl is shown to be highest and cell death at maximum cinnamon extract concentration of 1.65 mg/100μl is highest.
Further these phytochemicals together showed less protein content on cultured U-87 cells and displayed lower protein expression in gel band pattern when compared to their control. Thus positive phytochemical results have shown to play an important role in inhibiting cancer properties.
Author’s Contribution
Tina Mary George –Project performed, Analysis and written research article.
Prabha M*—Project plan, guidance for article writing and editing.
Mukta S Patil --Project performed.
Soumya Sakshi-- Project performed.
Shifa -- Project performed.
Abbreviations
BBB- Blood brain Barrier; DMEM- Dulbecco's Modified Eagle Medium; DMSO- Dimethyl sulfoxide; DPBS- Dulbecco's Phosphate Buffered Saline; ELISA- Enzyme-linked immunoassay; FBS- Fetal bovine serum; GBM- Glioblastoma Multiforme; MTT- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; NCCS- National Centre for Cell science; SDS-PAGE- Sodium Dodecyl Sulphate polyacrylamide gel electrophoresis; TMZ- Temozolomide; Trypsin-EDTA- Trypsin-disodium ethylenediaminetetraacetic acid; IC50- Inhibitory concentration
Conflict of Interest
The authors declare no conflict of interest.
Source of Funding
This research received no external funding.
Acknowledgements
Authors would like to thank Head of Department of chemical engineering Dr Archana and technical staff, Head of Department of Biotechnology Dr. Chandra Prabha, and technical staff Ramaiah Institute of Technology, Bengaluru, owner of Stroma Biotechnologies Pvt Ltd, Bengaluru.
References
- F Hanif, K Muzaffar, K Perveen, SM Malhi, SU Simjee. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac J Cancer Prev 2017. [Google Scholar]
- D Nizamutdinov, EM Stock, JA Dandashi, EA Vasquez, Y Mao, S Dayawansa. Prognostication of Survival Outcomes in Patients Diagnosed with Glioblastoma. World Neurosurg 2018. [Google Scholar] [Crossref]
- J Li, W Wang, J Wang, Y Cao, S Wang, J Zhao. Viral Gene Therapy for Glioblastoma Multiforme: A Promising Hope for the Current Dilemma. Front Oncol 2021. [Google Scholar] [Crossref]
- M E Davis. Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs 2016. [Google Scholar]
- MV Blagosklonny. Selective protection of normal cells from chemotherapy, while killing drug-resistant cancer cells. Oncotarget 2023. [Google Scholar]
- . Glioblastoma Multiforme - Symptoms, Diagnosis and Treatment Options. . [Google Scholar]
- N V Klinger, S Mittal. Therapeutic Potential of Curcumin for the Treatment of Brain Tumors. Oxid Med Cell Longev 2016. [Google Scholar] [Crossref]
- S Sadeghi, A Davoodvandi, M H Pourhanifeh, N Sharifi, R Arefnezhad, R Sahebnasagh. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur J Med Chem 2019. [Google Scholar] [Crossref]
- S Brahmachari, A Jana, K Pahan. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J Immunol 2009. [Google Scholar]
- J Sharifi-Rad, A Dey, N Koirala, S Shaheen, NE Omari, B Salehi, . Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front Pharmacol 2021. [Google Scholar] [Crossref]
- J Shaikh, M Patil. Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud 2020. [Google Scholar]
- . Phytochemical Screening and Antimicrobial Activity of “Cinnamon zeylanicum. Int J Pharm Res Innov 2020. [Google Scholar]
- C P Segeritz, L Vallier, M Jalali, FYL Saldanha, M Jalali. Basic Science Methods for Clinical Researchers. 2017. [Google Scholar]
- PF Ledur, GR Onzi, H Zong, G Lenz. Culture conditions defining glioblastoma cells behavior: what is the impact for novel discoveries?. Oncotarget 2017. [Google Scholar]
- Z Aminzadeh, N Ziamajidi, R Abbasalipourkabir, S Daei, S Helbi, A Moridnia. Antitumor Activities of Aqueous Cinnamon Extract on 5637 Cell Line of Bladder Cancer through Glycolytic Pathway. Int J Inflamm 2022. [Google Scholar] [Crossref]
- AB Nowakowski, WJ Wobig, DH Petering. Native SDS-PAGE: High Resolution Electrophoretic Separation of Proteins With Retention of Native Properties Including Bound Metal Ions. Metallomics 2014. [Google Scholar]
- J Redfern, M Kinninmonth, D Burdass, J Verran. Using Soxhlet Ethanol Extraction to Produce and Test Plant Material (Essential Oils) for Their Antimicrobial Properties†. J Microbiol Biol Educ 2014. [Google Scholar]
- M Prabha, TS Anusha. Esterase’s properties in commonly used Indian spices for Alzheimer’s disease model . J Biochem Tech 2015. [Google Scholar]
- R Chandrashekar, KK Angajala, Y Reddy, P Chaitanya, N Bhavani, J Pochampalli. Isolation of Gossypol and Analysis of Phytochemicals in Seed Extract of Bt and Non-Bt Varieties of Cotton. J Pharmacogn Phytochemistry 2013. [Google Scholar]
- U Joshi, R Wagh. Analysis of Phytochemical Compounds present in the bark extracts of Ehretia laevis Roxb. Int J Res Dev Pharm Life Sci 2018. [Google Scholar] [Crossref]
- . MTT Assay Protocol for Cell Viability and Proliferation. . [Google Scholar]
- P Kumar, A Nagarajan, PD Uchil. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb Protoc 2018. [Google Scholar] [Crossref]
- Introduction
- Materials and Methods
- Procuring of cell lines and other materials
- Cinnamon extraction from cinnamon verum
- Phytochemical analysis of cinnamon extract
- Maintenance of cell lines
- Sub Culturing
- Cryopreservation
- Determination of Cell Viability by MTT Assay
- Extraction and Separation of Protein by SDS PAGE
- Protein Estimation by Lowry’s Method
- Results and Discussions
- Extracting cinnamon from cinnamon verum
- Phytochemical analysis of cinnamon extract
- Determination of U87-MG Cell viability by MTT assay
- Extraction and Separation of Protein by SDS PAGE
- Protein Estimation by Lowry’s Method
- Conclusions
- Author’s Contribution
- Conflict of Interest
- Source of Funding
- Acknowledgements
