- Visibility 10.1k Views
- Downloads 304 Downloads
- Permissions
- DOI 10.18231/j.ijn.2023.028
-
CrossMark
- Citation
Methylcobalamin injection 1500 mcg SC route versus IM route: A randomized parallel comparative bioavailability study
- Author Details:
-
Nimesh P Modi *
-
Anil J Jaiswal
-
PNSS Vasu
Abstract
Background: Corona Remedies developed B-29 AQ PFS (Methylcobalamin Injection 1500 mcg) SC route for better patient compliance and ease of administration. The study outcome will explain pharmacokinetic behaviour of Methylcobalamin SC injection and non-inferiority over IM injection.
Materials and Methods: The study was conducted to compare bioavailability, safety and tolerability of two different Methylcobalamin Injection formulation 1500 mcg with SC versus IM route.
This was randomized, two treatment, parallel, comparative bioavailability study conducted on 24 normal, healthy, adult, human subjects.
The dosage of the investigational product (IP) was given either as a 1500 mcg SC injection into the abdominal muscle or as an intramuscular injection into the gluteal muscle (buttock region) following an overnight fast of at least 10 hours. To assess the plasma concentrations of methylcobalamin, a number of blood samples were collected both before and after the injection. Maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax) and the area under the concentration-time curve (AUC0-72) were compared. Pharmacokinetic (PK) parameters were statistically analysed using Statistical Analysis Software (SAS ®) Version 9.4 or above.
Results: The mean Tmax for methylcobalamin absorption from SC administration and IM injection was 1.38 hours and 1.49 hours, respectively. In terms of bioavailability, the SC injection is comparable to the IM injection (the ratio of population geometric means for the SC and IM routes is 103.62 for AUC0-72). In this study, a higher Cmax for the SC route than the IM route was found (57.01 versus 45.82). Based on log transformed primary PK parameters (Cmax and AUC0-72), the geometric least squares mean ratio was observed >80.00%. According to safety evaluations, both therapies were safe and well-tolerated.
Conclusions: Methylcobalamin is a safe and well-tolerated alternative to the current one that is non-inferior to IM route and faster in absorption after SC route.
Introduction
The best vitamin B12 analogue for treating or preventing the problems linked with vitamin B12 deficiency is methylcobalamin. [1]
The creation of DNA, the development of red blood cells, and neurological processes are all significantly influenced by vitamin B12. A lesser than 148 pmol/L (200 pg/ml) was used by the National Health and Nutrition Examination Survey to identify biochemical vitamin B12 deficiency. [2], [3]
It is unknown how common vitamin B12 insufficiency is in the general population. However, it seems that the incidence rate rises with age. [4]
The intramuscular injections are uncomfortable, unpleasant, and frequently followed by unfavourable reactions (such as abscess formation, erythema, and hematoma). [5], [6]
The SC injection, however, was created to be self-administered, to improve patient compliance, and to be cost-effective and easy to use. Both patients and medical professionals typically appreciate SC administration as a safe and effective dosage alternative. [7]
A unique Methylcobalamin SC injection (1500 mcg) has been formulated by Corona Remedies Pvt. Ltd. considering the benefits of the formulation.
The goal of this study was to compare the bioavailability of methylcobalamin SC injection and IM injection, as well as the safety and tolerability of both formulations, in healthy, adult human volunteers in a randomized, single-dose, open label, parallel design study. The study outcome will explain pharmacokinetic behaviour of SC injection and non-inferiority over IM injection of methylcobalamin.
Materials and Methods
Raptim Research Pvt. Ltd. carried out the clinical phase. The study received IEC (Independent Ethics Committee) approval and it was carried out in accordance with the World Medical Association's Declaration of Helsinki, ICH (International Conference on Harmonization) E6(R2) "Guideline for Good Clinical Practice," and ICMR (Indian Council of Medical Research) regulations.
Selection of subjects
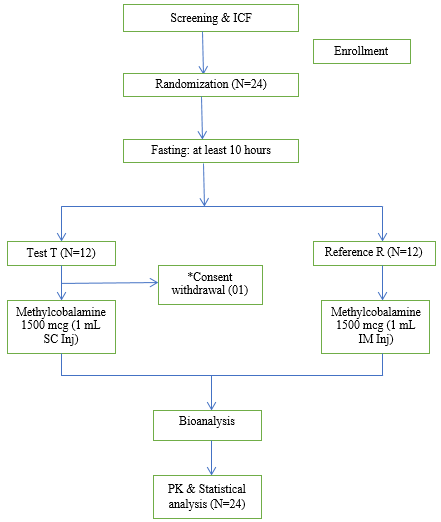
The study included twenty-four (24) healthy adult male human individuals with body mass indices between 18.50 and 29.99 kg/m2 and age ranging from 18 to 45 (both inclusive). Prior to enrollment, all subjects gave their written informed consent to participate in the study. They were also given the option to withdraw at any point while the study was ongoing. Subjects were only considered if their hematology, biochemistry, urine tests, and ECG results were consistent with their clinical findings. Subjects who were known to be allergic to methylcobalamin were eliminated. The study protocol's inclusion and exclusion criteria were followed by every subject.
Study procedure
This was parallel comparative bioavailability study designed as randomized, single dosage and open labelled. To find the significant variation in PK behaviour between two formulations, a sample size of 24 subjects was deemed acceptable. From February 25 until March 14, 2023, the clinical phase took place. Between 36.00 hours before the treatment and 72.00 hours after the dose, the subjects were kept in a clinical facility. Each subject received either a 1.0 mL (1500 mcg) SC injection of methylcobalamin made by Corona Remedies Pvt. Ltd. in the abdominal region or an intramuscular injection in buttock region, according to the randomization schedule, which was created using SAS ® software (SAS Institute Inc., USA) version 9.4 or higher.
Safety assessment
During the in-house phase, measurements of vital signs (blood pressure and pulse rate) and well-being were made at -25.00, -11.00 (±45 minutes of scheduled time), 0.00 (within 2.00 hours) prior to dosing, and at 2.00, 5.00, 11.00, 25.00, 37.00, 49.00, and 61.00 hours post-dose (±45 minutes of scheduled time). During the in-house phase, body temperature was measured at -25.00, -11.00 (±45 minutes of the scheduled time), 0.00 (within 2.00 hours), and at 11.00, 25.00, 37.00, 49.00, and 61.00 hours after the dosage (±45 minutes of the scheduled time). At screening and post study, ECG, Biochemistry, Hematology, and Urinalysis were performed.
PK Blood samples (each of 5.0 mL) were withdrawn at -24.00 and -12.00 (±5 minutes), 0.00 (within 2.00 hours), 0.17, 0.33, 0.50, 0.67, 0.83, 1.00, 1.25, 1.50, 1.75, 2.00, 2.50, 3.00, 4.00, 6.00, 8.00, 12.00, 16.00, 24.00, 36.00, 48.00 and 72.00 hours post-dose in K3EDTA. To separate the plasma from the blood, blood samples were centrifuged at 4000 RPM for 10 minutes at 5°C ±3°C. Within 60 minutes of collection, the plasma samples were divided roughly in half of equal volume into pre-labeled polypropylene amber coloured tubes and stored in the deep freezer kept at -70°C (Range: -60°C to -85°C).
Estimation of plasma methylcobalamin
An approved LCMS-MS technique was used to measure the levels of methylcobalamin in the blood. For all PK and statistical analyses, concentrations below the Lower Limit of Quantification (0.50 ng/mL) were recorded as LLOQ and treated as zero. It was calibrated between 0.50 and 50 ng/mL. The randomization schedule was kept blinded from the bioanalysts until the analysis was finished.
Pharmacokinetic and statistical analysis
The non-compartmental Phoenix ® WinNonlin ® Software, Version 8.3.5 or higher (Certara USA, Inc.), was used to calculate the primary pharmacokinetic parameters Cmax, AUC0-72, and secondary PK parameter Tmax. Utilizing SAS ® (SAS Institute Inc., USA) Version 9.4 or higher, statistical analysis was carried out. ANOVA was carried out on the natural log-transformed PK parameters Cmax and AUC0-72 for methylcobalamin in accordance with Schuirmann's two one-sided testing technique for bioavailability.
Results
Subject disposition
Twenty-four (24) subjects who met the qualifying requirements took part and completed the study.
One subject withdrew from the study before pre-dose blood sample collection due to personal reason, which was replaced by standby subject. [Table 1] provides the baseline demographic information for the research populations, and [Figure 1] describes the subject disposition.
Safety
One adverse event (Lymphocyte decreased) was reported in post study safety assessment in subject treated with reference methylcobalamin IM injection. This adverse event was unlikely, mild in nature and subject was lost to follow up. In all subjects who participated, there were no clinically significant findings in the vital signs or ECG recordings. No Serious Adverse Event (SAE), including any AE/SAE unique to the SC route, was reported in the study. As a result, both reference and test formulations were safe and well tolerated.
Pharmacokinetics
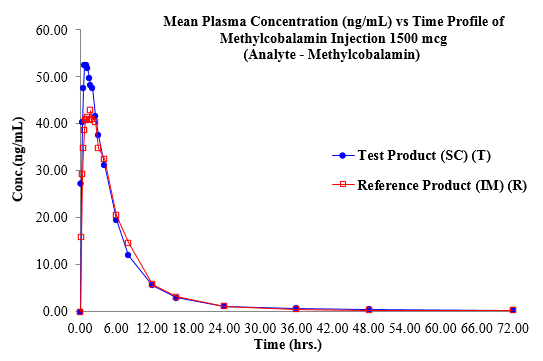
[Figure 2] displays the plasma Metylcobalamine concentration vs. time profile for each time point for both treatments. [Table 2] summarizes the plasma concentration at each PK time point.
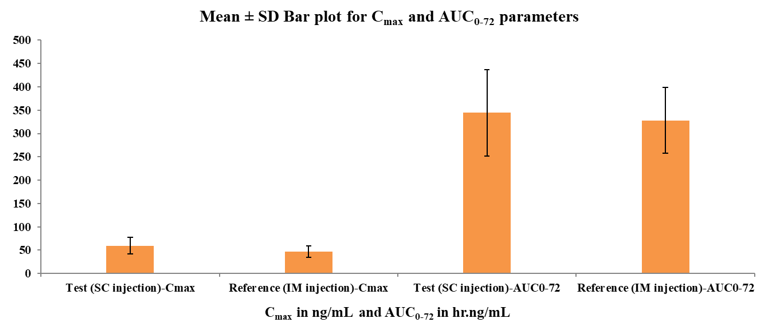
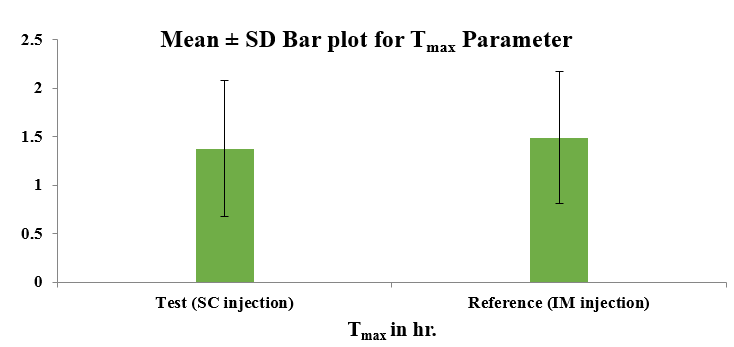
[Table 3] displays the computed mean PK parameters of the methylcobalamin 1500mcg test (SC injection) and reference (IM injection). The mean and standard deviation of the plasma levels of methylcobalamin for the test (SC) and reference (IM) were 59.44 ± 17.73 ng/ml and 47.10 ± 11.96 ng/ml, respectively. When compared to IM injection, SC administration produced a peak concentration earlier, with a mean Tmax of 1.38 hours (for SC) versus 1.49 hours (for IM).
In [Table 4], the geometric least squares means (%) for the test and reference products for the Ln-transformed data are summarized.
methylcobalamin bioavailability was higher following SC injection than IM administration, with test/reference ratios of geometric least square means for Cmax and AUC0-72 data having Ln-transformed values of 124.43% and 103.62%, respectively. The mean maximum concentration and AUC0-72 of methylcobalamin in plasma following SC injection administration were larger than those following IM injection.
|
Variables |
Age (Years) |
Weight (kg) |
Height (cm) |
BMI (kg/m2) |
Gender |
|
Mean ± SD |
33.69±5.80 |
62.04±10.46 |
166.92±7.01 |
22.20±2.95 |
Male |
|
Median |
34.00 |
60.09 |
168.00 |
21.80 |
|
Time (hours) |
Test Product (SC) (T) |
Reference Product (IM) (R) |
|
-24.00 |
0.00 |
0.00 |
|
-12.00 |
0.00 |
0.00 |
|
0.00 |
0.00 |
0.00 |
|
0.17 |
27.24 |
15.92 |
|
0.33 |
40.28 |
29.30 |
|
0.50 |
47.66 |
34.84 |
|
0.67 |
52.54 |
38.68 |
|
0.83 |
52.51 |
40.67 |
|
1.00 |
52.37 |
40.93 |
|
1.25 |
51.89 |
41.33 |
|
1.50 |
49.74 |
40.74 |
|
1.75 |
48.31 |
42.87 |
|
2.00 |
47.67 |
41.07 |
|
2.50 |
41.63 |
40.44 |
|
3.00 |
37.55 |
34.82 |
|
4.00 |
31.27 |
32.53 |
|
6.00 |
19.55 |
20.38 |
|
8.00 |
12.03 |
14.63 |
|
12.00 |
5.50 |
5.79 |
|
16.00 |
2.93 |
3.15 |
|
24.00 |
1.19 |
1.12 |
|
36.00 |
0.68 |
0.50 |
|
48.00 |
0.42 |
0.31 |
|
72.00 |
0.27 |
0.17 |
|
Pharmacokinetic Parameters |
Test Mean ± SD |
Mean ± SD |
|
Cmax (ng/mL) |
59.44±17.73 |
47.10±11.96 |
|
AUC0-72 (ng*hr/mL) |
344.44 ±92.44 |
327.87±70.51 |
|
Tmax (hr) |
1.38 ±0.70 |
1.49±0.68 |
|
Parameters |
Ln(Cmax) |
Ln(AUC0-72) |
|
Test Geo LSM |
57.01 |
332.62 |
|
Ref Geo LSM |
45.82 |
321.01 |
|
Ratio (%) |
124.43 |
103.62 |
|
CI_90_Lower |
102.57 |
86.92 |
|
CI_90_Upper |
150.94 |
123.51 |
|
Power (%) |
46.44 |
54.23 |
|
Inter_CV% |
28.09 |
25.45 |
Discussion
The goal of this randomized, single-dose, open-label, parallel comparative bioavailability study was to compare the safety and non-inferiority of injectable methylcobalamin administered via the SC route to that administered via the IM route.
For both the Test and Reference treatments, 1500 mcg of methylcobalime were given to the participants. It was thought that baseline adjustment for pharmacokinetic data would allow for a more realistic comparison of the effectiveness of different formulations. Due to zero pre-dose concentrations, baseline correction was not relevant in this investigation.
In this study, it was discovered that the bioavailability of methylcobalamin SC injection and IM injection were equivalent. Commercial oral B12 pills have been found to have a low bioavailability.[8] In comparison to the IM route, the SC route formulation of methylcobalamin may promote quicker absorption and effective therapy for multivitamin deficiency. Additionally, SC route is more practical than IM injections and does not require medical specialists for administration. As an alternative to both oral and intramuscular preparations, methylcobalamin SC injection can be used to treat vitamin B12 insufficiency.
This study revealed significant pharmacokinetic difference between SC and IM routes of methylcobalamin in healthy adult human participants. After SC administration the Cmax and AUC0-72 were higher, and Tmax was reached quicker (1.38 ± 0.70 hours), compared with IM administration (1.49 ± 0.68 hours). The estimated ratio of (Ln transformed) geometric least square means for primary PK parameters of methylcobalamin is > 80%. The corresponding bioavailability indicator; rate (Cmax) and the extent of absorption (AUC0-72) in this study were observed comparatively higher in Test formulation than Reference formulation. The results suggest that the absorption with Test formulation was higher than Reference formulation.
For both the SC route and the IM route, the Cmax and AUC0-72 values found in this study were comparable.
These findings suggest that the therapeutic dose for (1500 mcg) SC and IM injections has a comparable bioavailability, suggesting a potential alternative for the treatment of vitamin B12 deficiency.
Methylcobalamin is known to be safe, and several countries throughout the world have approved a 1500 mcg dose for IM injection. This is also confirmed by the current investigation, which demonstrated that single dosages administered via the SC and IM routes were well tolerated and safe. Throughout this investigation, no severe adverse effects were observed.
Injections intramuscularly have a number of drawbacks. Health care professionals are typically required to administer injections because they can be painful, expensive to administer, and associated with adverse reactions (such as intramuscular hematoma) as well as poor treatment adherence and bleeding, particularly in elderly patients who are frequently prescribed anticoagulant medication. The latter significantly increases the treatment burden. Both patients and the healthcare system as a whole would benefit from a ease of administration and safe procedure. [9]
Considering these issues, SC route is now being considered a choice for methylcobalamin administration. SC route has several advantages, for example, it is having no administration cost, results in high patient satisfaction, does not require a medical professional to administer, is not much painful, and does not result in injection related injury.[10]
Treatment with methylcobalamin can prevent irreversible neurologic damage.[11] Local subcutaneous injection of methylcobalamin appears to be more effective than systemic administration and generally safe for older individuals.[12] Local subcutaneous injection of methylcobalamin is more effective than systemic administration for relieving zoster-related pain with acute herpetic neuralgia. [13] Moreover, SC formulation of Methylcobalaime already exists in market since long back. With the due help of the present study.[14], [15] medical fraternity come to know the comparative treatment benefits to patients, compliance, ease of administration and suitability of prescription to select preferred dosage forms.
Conclusion
Methylcobalamin 1500 mcg when administered subcutaneously is faster in absorption, non-inferior to IM route and bioavailability of both routes was comparable. It can be self administered, safe and effective. Thus it could offer an alternative mode of administration against the current IM injectable drug available in the Indian market for various indications.
Thus methylcobalime SC route has the main advantage with regards to the ease of administration without medical assistance and preferably more patient compliance compared to other route of administration.
Conflict of Interest
None.
Source of Funding
Investigational Product and financial support was provided by Corona Remedies Pvt. Ltd.
Acknowledgement
Investigational Product and financial support was provided by Corona Remedies Pvt. Ltd.
Clinical conduct, bio-analysis and statistical analysis were carried out by Raptim Research Pvt. Ltd.
Author thanks and appreciates the scientific team of Raptim Research Pvt. Ltd. for conceptualization and manuscript writing of this original research article.
References
- Amrita S, Hrishikesh K, Rahul DM, Sidharth S, Anirban G, Ashis D. A randomized, open labeled study comparing the serum levels of cobalamin after three doses of 500 mcg vs. a single dose methylcobalamin of 1500 mcg in patients with peripheral neuropathy. Korean J Pain. 2018;31(3):183-90. [Google Scholar]
- Zagar B, Longyhore D. Evaluating the association between vitamin B12 deficiency and peripheral neuropathy in patients with diabetes. Int J Med Pharm. 2014;2(2):1-10. [Google Scholar]
- Briani C, Dalla TC, Citton V, Manara R, Pompanin S, Binotto G. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521-39. [Google Scholar] [Crossref]
- Oh R, Brown D. Vitamin B12 deficiency. Am Fam Physician. 2003;67(5):979-86. [Google Scholar]
- Greenblatt DJ, Allen M. Intramuscular injection-site complications. JAMA. 1978;240(6):542-4. [Google Scholar]
- Sartorius G, Fennell C, Spasevska S, Turner L, Conway AJ. Factors influencing time course of pain after depot oil intramuscular injection of testosterone undecanoate. Asian J Androl. 2010;12(2):227-33. [Google Scholar] [Crossref]
- Beate B, Wolfgang R, Johannes S. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs. 2018;32(5):425-40. [Google Scholar] [Crossref]
- Castelli M, Wong D, Friedman K, Riley M. Pharmacokinetics of oral cyanocobalamin formulated with sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): an open-label, randomized, single-dose, parallel-group study in healthy male subjects. Clin Ther. 2011;33(7):934-45. [Google Scholar] [Crossref]
- Walraven V, Austin P, Naylor C. Vitamin B12 injections versus oral supplements. How much money could be saved by switching from injections to pills?. Can Fam Physician. 2001;47:79-86. [Google Scholar]
- Ayse T, Zeynep C. Comparison of Sublingual and Intramuscular Administration of Vitamin B12 for the Treatment of Vitamin B12 Deficiency in Children. Rev Invest Clin. 2020;72(6):380-5. [Google Scholar]
- Carol RP. Revisiting Vitamin B12 Deficiency: A Clinician’s Guide for the 21st Century. Pract Gastroenterol. 2018. [Google Scholar]
- Gang X, LZ, FY, TW, XGX. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med. 2013;14(6):884-94. [Google Scholar] [Crossref]
- Gang X, Site X, Wei-Zhen T, Gang X, Chao C, Jie X. Local Injection of Methylcobalamin Combined with Lidocaine for Acute Herpetic Neuralgia. Pain Med. 2016;17(3):572-81. [Google Scholar] [Crossref]
- . New Drugs and Clinical Trials (Amendment) Rules, 2021 [Gazette notification G.S.R.227 (E), dated 19.03.2019 & G.S.R.605 (E), dated 31.08.2021], Ministry of Health and Family Welfare, Government of India. . . [Google Scholar]
- . Ethical Guidelines for Biomedical Research on Human Participants, Indian Council of Medical Research. . 2017. [Google Scholar]
How to Cite This Article
Vancouver
Modi NP, Jaiswal AJ, Vasu P. Methylcobalamin injection 1500 mcg SC route versus IM route: A randomized parallel comparative bioavailability study [Internet]. IP Indian J Neurosci. 2023 [cited 2025 Sep 30];9(3):141-147. Available from: https://doi.org/10.18231/j.ijn.2023.028
APA
Modi, N. P., Jaiswal, A. J., Vasu, P. (2023). Methylcobalamin injection 1500 mcg SC route versus IM route: A randomized parallel comparative bioavailability study. IP Indian J Neurosci, 9(3), 141-147. https://doi.org/10.18231/j.ijn.2023.028
MLA
Modi, Nimesh P, Jaiswal, Anil J, Vasu, PNSS. "Methylcobalamin injection 1500 mcg SC route versus IM route: A randomized parallel comparative bioavailability study." IP Indian J Neurosci, vol. 9, no. 3, 2023, pp. 141-147. https://doi.org/10.18231/j.ijn.2023.028
Chicago
Modi, N. P., Jaiswal, A. J., Vasu, P.. "Methylcobalamin injection 1500 mcg SC route versus IM route: A randomized parallel comparative bioavailability study." IP Indian J Neurosci 9, no. 3 (2023): 141-147. https://doi.org/10.18231/j.ijn.2023.028
