Introduction
Autism spectrum disorder (ASD) is a neurodevelopment disorder characterized by a deficit in social interaction, verbal and nonverbal communication starting from early infancy. The prevalence of ASD is estimated to be about 1-2% and is on an increasing trend.1, 2, 3 ASD has a higher risk of epilepsy with prevalence ranging from 5%-46%. Conversely, ASD has increased in the population with epilepsy (32%).4 However, at present there is a scarcity of data to help in predicting, which children with autism will develop epilepsy/EEG abnormalities and to what extent cognition would be affected.5
Epileptiform activities are common in cases with active epilepsy but are rare (1-4%) in healthy children.6, 7 Past studies have shown that interictal epileptiform discharges (IED) varied from 6%-30% of ASD patients.8, 9 Some studies have also shown epileptiform activity in the EEG of approximately 30% of ASD patients without seizures.10, 11 Mulligan and Trauner,12 postulated that the severity of autistic symptoms may be associated with higher chances of epileptiform changes. A study has shown that IED in autistic children indicates more severe diseases, severe behavior problems, and severe social deterioration.13
Some studies have also revealed that there is no significant association between ASD and IED. Tetsu et al. did not find a relationship between IED and ASD in children proposing an epiphenomenon or compensatory changes.14
Materials and Methods
The aim of this cross-sectional study was to describe the prevalence of electroencephalographic characteristics in children with ASD. To compare the spectrum of various EEG changes in epileptic and non-epileptic individuals and various clinical features and severity in ASD.
A total of 140 consecutive cases of autism spectrum disorder visiting the neuro-developmental clinic at a tertiary care centre and who are diagnosed with Diagnostic and Statistical Manual-V (DSM-V) criteria were included in the study after taking consent. Preliminary information like demographic data, age, sex, history, and clinical profile was collected from all the participants with the help of their caregivers. Details regarding the history of seizures, their types, frequency, age of onset and therapeutic measures, neuropsychiatric and neurobehavioral symptoms such as sleep disturbance, hyperactivity, aggressiveness, and language regression were also recorded. Indian Scale for Assessment of Autism (ISAA)15 score was entered in Case record form (CRF). All the patients who are diagnosed to have an Autistic spectrum disorder had undergone electroencephalographic (EEG) evaluation as a part of institutional protocol, for the presence of any abnormal activity. All the EEGs were reported by experts (double-blinded) in pediatric Neurology. EEG variables were studied and recorded on CRF (Table of EEG variables). Comparison between different groups regarding categorical variables was tested using X2 test. When more than 20% of cells have expected counts < 5 correction for X2test was conducted using Fisher’s exact test. If it reveals normal data distribution parametric tests were applied and if the data are abnormal non-parametric tests were applied. The correlation between two quantitative variables was assessed using the Pearson coefficient. Prevalence was calculated with a confidence limit of 95%. All analysis was done using SPSS 20.0 and a p-value of < 5% was considered significant.
Results
The demographic characteristics of the cohort of this study are described in Table 1. The study group had male predominance with a male: female ratio of 4.8:1. The most common age group in ASD cases was 4-6 years. Mean age at which the parents noticed symptoms was earliest at 1 year with the latest of 8 years. The mean age of the study cohort was 6 ± 2.75 years. According to ISAA scoring, the majority (n=92) of ASD cases had mild symptoms, and 48 cases were suffering from moderate symptoms. The neuro-psychological symptoms noted in the candidates were as follows- developmental delay, stereotypes, attention deficit hyperactivity disorder (ADHD), aggression/ tantrums, language regression, and sleep disturbances in 94%, 78%, 65%, 47 %, 16%, and 6% cases respectively.
Seizure was noted in 17% of cases. Generalized tonic-clonic convulsion is the most common seizure type (83% cases) followed by focal seizure with impaired awareness (17% cases) . EEG changes were seen in 45% (n=63) of cases of ASD. On studying the EEG background, it was noted that 98% of cases had symmetrical backgrounds, while only 2% had asymmetry. It was observed that most cases had normal with good anterior-posterior gradient (91% cases) and 9% cases showed slow background activity. (Table 1)
Table 1
Demographic details of study profile
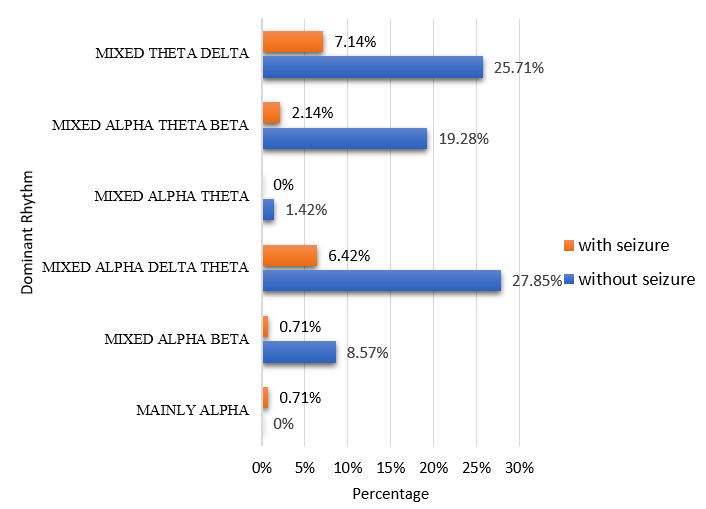
The dominant background waveforms in the study were intermixed alpha, delta, theta (34.28%) followed by mixed theta, delta (32.85%), mixed alpha, theta, beta (21.4%), mixed alpha, beta (9.28%). Mixed alpha theta contributed to 1.4% while mainly alpha rhythm was present in less than 1% of cases in the study. (Table 2)
Table 2
EEG Background in ASD cases with and without seizure
In this study, 36% of cases had paroxysmal activity. Focal paroxysmal activity was seen in 35 cases while generalised activity was seen in 8 cases. However, 8 cases displayed both focal and generalized paroxysmal activity. In focal paroxysmal activity, bilateral (n=29) spikes (n=27) were the most common. Focal paroxysmal activity was predominantly localized to the frontal region. In Generalised paroxysmal activity, symmetrical (n=11) spikes (n=10) were predominantly seen. (Table 3)
Table 3
Comparison of cases of ASD with and without seizure according to paroxysmal activity (spikes and sharp waves)
Comparison of ASD cases with and without seizure
Of the total 140 cases, 17% (n=24) had seizures. Unlike the cases of ASD with seizure, where most cases were in the age group 7-9 years, the cohort without seizure had maximum cases in the age group 4-6years. Male predominance was noted in cases of ASD. However, the seizure was found to be more common in females (p-value- 0.001) with M: F ratio of 1.2:1. (Figure 1)
The frequency of neuro-psychological symptoms noted in the candidates without seizure was as follows, developmental delay in 93% of cases, stereotypes in 77% of cases, ADHD in 66% cases, aggression/ tantrums in 47% of cases, language regression in 18% cases, and sleep disturbances in 9% cases. Similarly in the cohort with seizures, the most common neuro-psychological symptoms were developmental delay and stereotypes followed by ADHD, aggression/tantrums, sleep disturbances, and language regression in 100%, 83%, 63%, 46%, 29%, 4% cases in the given order. Sleep disturbance was significantly associated with a cohort of ASD with seizure (p value-0.009).
In the cohort without seizures, about 68% of cases were of mild ASD whereas in the cohort with seizures almost equal numbers of mild (54%) and moderate (46%) ASD.
On the assessment of EEG background in ASD cases without a seizure, 1% of cases had asymmetry and 9% of cases had a slow background. The most dominant rhythm in cases without seizure was mixed alpha delta theta (28%) followed by mixed theta delta (26%) and mixed alpha theta beta (19%). Other patterns noted were mixed alpha-beta (9%), mixed alpha theta (1%), and mainly alpha (1%). A similar pattern was noted in cases with seizure, with 7% cases of mixed theta delta, 6% cases of mixed alpha delta theta, 2% cases of mixed alpha theta beta, and 1% cases of mixed alpha-beta. (Figure 1)
In this study, 51 cases (36.4%) had paroxysmal epileptiform activity, of which 33 cases (24%) had no seizure. In cases with focal paroxysmal activity, spikes (epileptic) i.e. 13% were more common than sharp waves (non-epileptic) i.e. 6% even in cases without a seizure. It was also noted that bilateral paroxysmal activity (14%) was more than unilateral activity (6 %) in cases without seizure with significant statistical association (p-value -0.030). In ASD cases with generalized paroxysmal activity; sharps waves were seen in 4% of cases without seizures while spikes were seen in 4% of cases with seizures. More asymmetrical generalized paroxysmal activity was seen in 4% of cases without a seizure, and symmetrical events in 5% of cases with a seizure which is statistically significant (p-value -0.023) (table-3)
Discussion
The higher prevalence of epilepsy and/or epileptiform EEG abnormalities in children with ASD may point toward the underlying neurological abnormality. However, there are no conclusive data to help prediction of epilepsy or EEG abnormalities in these children. There is a lack of strong evidence in the literature to know to what degree behavior, cognition, and phenotypic characteristics in autistic children are direct consequences of underline epilepsy or EEG abnormalities.
Studies in the past have documented an increased prevalence of ASD in boys compared to girls. (Kanner 1968).16 In this study, male predominance was noted with the male: female ratio of 4.8:1. In the study by Elkholy et al. on 30 ASD cases 80% were male vs 20% were female with male to female ratio of 4:1.17 The postulates suggest that males may have a low threshold for expression of the disorder compared to females. (Gillberg et al., 1991).15 Others suggest that high foetal testosterone is associated with autistic traits in toddlers and older children. (Auyeung et al.2010).18
Idiopathic ASD is believed to be due to multigenic complex genetics, however, single-gene defects are also known to be associated with both ASD and seizure disorder.19 In previous studies the Pathophysiologic basis for increased seizures in ASD points towards defects in postsynaptic functions of neurons and abnormal signalling.20, 18 In this study 17% of ASD cases had seizures, in which male: female ratio was 1.2:1. This finding was statistically significant with seizures being more common in female children with ASD. ASD is associated with an increased risk of epilepsy with prevalence ranging from 5%-46%.21 Conversely, ASD is also increased in the population with epilepsy (32%).4 In a few studies, the prevalence of seizure in ASD is higher in females compared to males with the proportion being 1.6-4:1.19, 22, 23, 24
All types of seizures have been documented to be associated with ASD- focal seizures with impaired awareness, absence, and generalized tonic-clonic are more frequently found.20, 25, 26 In this study most common seizure type was generalized tonic seizure, followed by focal seizure with impaired awareness. In the study by Samra et.al (2017), sixty patients (42 males and 18 females) diagnosed with ASD were enrolled. 30.0% had Seizures of which 55.6% had generalized tonic-clonic seizures, 22.2% had Myoclonic seizures, 11.1% Febrile convulsions, and 11.1% had Complex focal convulsions. 27
The central mechanism in the development of ASD is impairment in connectivity and in synaptic plasticity. Frontal and temporal lobes are markedly affected in ASD. Pathological and neuroimaging studies have shown the involvement of the amygdala which is strongly associated with social and aggressive behavior problems. 28, 29 The associated neuro-psychological symptoms with ASD in this study were ADHD, aggression/tantrums, language regression, stereotype behavior, sleep disturbances, and developmental delay which often co-existed in a particular patient. Cases without seizures had a predominantly developmental delay (93%), motor stereotypes (77%), and hyperkinetic behavior (66%), which is similar to cases with seizures. Of these only sleep disturbances were found to be associated with seizures in ASD children with significant statistical value (p-value -0.009). In a study done by Elkholy et al. ADHD was seen in 20% and sleep disturbances were seen in 16.7% of cases.17 Samra et.al (2017) Regarding neuropsychological history among the study subjects: aggressive behaviors, hyperactivity, and delayed mental development were the most prevalent comorbidities with sleep disturbances observed in fewer.30
Rates of EEG abnormalities nearing 60% have been reported by other studies and investigators, who propose that EEG abnormalities may play a causal role in autism (Spence and Schneider, 2009).31 In this study, out of 140 cases, 45% cases had abnormal EEG. Lewine et al. (1999) reported a 64.7% frequency of EEG changes in autistic children.32 Prevalence of EEG abnormalities was seen in 38.3 to 60.8% of cases and in 20% of cases in the study by Tamarah et al. and Tuchmann et.al respectively even in the absence of seizure.33, 34
The EEG characteristics noted in this study were as follows, 36.4% of cases had paroxysmal epileptic or nonepileptic discharges, 2% of cases had an asymmetrical background and 9% of cases had slow background EEG, and few of the EEGs had overlapping above features. It was noticed that 2 cases had an asymmetrical background and 12 cases had slow background on EEG in ASD cases without seizure. A study by Elkholy et al has revealed symmetrical background EEG activity in 96.7% of cases with ASD. Also, 93.3% of cases had well-formed background EEG, and 6.7% had slow-for-age EEG activity.17
The dominant background EEG rhythm in the study was mixed alpha, delta, and theta followed by mixed theta delta mixed alpha theta beta. A mixed pattern was observed since the majority of EEG was done awake followed by sleep. The most dominant rhythm in cases without seizure was mixed alpha delta theta (28%) while in cases with seizure, the most common dominant rhythm was mixed theta delta (7.14%). Dominant rhythm on background EEG in ASD patients in a study by Elkholy et al. showed mixed alpha and theta as the most common EEG finding comprising 46.7% followed by mainly alpha (26.7%) and mixed theta and delta (16.7%).17
In a previous study by Samra et.al (2017), regarding EEG findings among study participants: 41.7% had slow waves, 41.7% had Focal epileptogenic activity, 8.3% had generalized epileptogenic activity and 8.3% had mixed; slow waves with focal epileptogenic activity.30 In this study, the prevalence of paroxysmal activity on EEG was found to be 36% cases, of which the most common was focal EEG activity seen in 31% of cases. In all the ASD cases with abnormal EEG, the Most common focal EEG activity were bilateral frontal sharp waves and bilateral frontal spikes and temporal spikes/sharp waves. Total of 11.42% cases had generalized EEG activity of which symmetrical spike-wave complex were more common than sharp wave complexes. A similar observation was reported by Hughes and Melyn (2005) in their study where epileptiform EEGs were more common than non-epileptiform abnormalities. 35 Also, the frontal predominance of EEG abnormality in our study was similar to research by Hara (2007) and Yasuhara (2010), which reported that paroxysmal EEG activities in the frontal area were common abnormalities in autism, which could be related to frontal lobe dysfunction in ASD.36, 37
Some studies have also shown epileptiform EEG discharges in approximately 30% of ASD patients without clinical seizures. 10, 11 On comparison of ASD cases with and without a seizure, similarity was noted in the form of the predominance of bilateral spikes/sharp waves in focal paroxysmal activity in both cohort. However, in Generalised paroxysmal activity, asymmetrical sharp waves (non-epileptic) were more common in cases without seizure and symmetrical spikes (epileptic) were more prevalent in cases with seizure.
Contrary to our observations, Elkholy et al. reported EEG changes were equal with 50% generalized and 50% focal, with the majority focal abnormalities in the centrotemporal area (54.5%) and in generalized abnormalities, 91% were symmetrical spike-wave complexes and about 9% asymmetrical bursts of poly-spikes.17 Chez et al. (2006) in a study on 1266 ASD children found abnormal EEG in 64.7% they also detected the most common was the right temporal region followed by bitemporal and generalized epileptiform activity and an equal percentage for remaining locations. 38 Lewin et al. and Tuchman et al. reported that the localization of EEG abnormalities in ASD children is variable. 32, 34 These included centrotemporal spikes to similarities to benign focal epilepsies.
This study was performed at a single centre with an adequate sample size with detailed EEG assessment in ASD cases by an expert but since this was a cross-sectional study, follow-up EEG was not feasible, to assess the number of cases with EEG abnormality progressing to epilepsy. Also, the clinical-psychological and neuro-radiological correlation could not be done in all, to evaluate its correlations and support any causal relationship between behavioral symptoms and EEG changes.
Conclusion
To summarize, Seizure and ASD are often seen co-existing in patients. On EEG in ASD patients, focal paroxysmal activity was the most common finding. The epileptic form discharges were predominantly localized to the frontal area, which may help to hypothesize a correlation between the EEG changes and behavioral disorders in these children. EEG changes were noted even in the case of ASD without a seizure. There is a paucity of data on the EEG at baseline and follow-up EEG in children with ASD without a history of seizures. Also, it is important to determine whether these two phenomena i.e. EEG changes and ASD are associated: If an association is noted pharmacological treatments could be tried to minimize the negative impact of epileptiform discharges on patients’ cognition and behavior and if no association is observed, patients can avoid the associated risks with treatment. The first year of life is the period with the greatest neurological development in life hence ASD needs prompt treatment to avoid the negative outcomes. Therefore, if the association exists, early intervention could have a strong impact on prognosis.