Introduction
Epilepsy affects more than 50 million people worldwide. 1 Over 20 antiepileptic drugs (AEDs) are available and approved for the treatment of different types of seizures. However, about one-third of these patients do not respond to AED treatment. 2, 3
In recent years, the choice of available AEDs has expanded with the approval of many newer drugs such as lacosamide, rufinamide, perampanel (Fycompa), and eslicarbazepine acetate. These AEDs have been approved by the US Food and Drug Administration (FDA) for adjunctive treatment of focal (partial-onset) seizures. 4, 5, 6, 7 with perampanel also approved for treatment of primary generalized tonic–clonic seizures. 5
Brivaracetam (BRV) is another new AED, approved in 2016 by the FDA for adjunctive treatment of focal seizures in patients aged ≥16 years with epilepsy. 8 BRV is a member of the racetam class of drugs, related to piracetam and the AED levetiracetam (LEV), and is the first selective ligand for SV2A. 9
BRV is extensively metabolized, primarily by hydrolysis of the acetamide group to the carboxylic acid metabolite by an amidase, 10, 11 followed by hydroxylation by cytochrome P450 (CYP2C9) to form a hydroxy-acid metabolite. β-Oxidation of the propyl side chain, mainly by CYP2C19, forms a secondary pathway. The three main metabolites (acid, hydroxy, and hydroxy acid) are not active. More than 95% of the BRV dose is eliminated in the urine within 72 hours, 8.6% is eliminated unchanged, and the rest is excreted as metabolites. Mean half-life is around ~9 hours and plasma clearance is 3.4 L/hour following a single BRV 50 mg oral dose in healthy participants. 12
BRV has 15- to 30-fold higher affinity for SV2A than LEV. 9
This study was conducted to assess data on the effectiveness of brivaracetam in patients with epilepsy in everyday clinical practices.
Materials and Methods
This study was designed to collect data on the effectiveness of brivaracetam in patients with epilepsy over a period of 1 year, with an aim to establish a correlation with LEV in everyday clinical practices.
The sampling technique was based on an opportunity principle, interviewing patients that visit the neurology OPD or inpatients of the hospital during the designated time frame.
Patients were interviewed for various parameters which included type of seizure, seizure frequency, drug dosage used, side-effects associated with the drug. Interviews were done in the hospital, whereas the final interview was planned to be done in person or telephonically, whichever feasible.
After the initial interview, each patient was contacted regularly at intervals over a course of 1 year till the final interview. This was done to test the efficacy of BRV in different patient subgroups. The method of obtaining information was again through interviews; however, this was done over-scheduled phone calls for convenience (most final ones - some initial ones were done via hospital visits).
These interviews were combined and then quantified for a clear progression of the results.
Results
The study was conducted on 35 patients (15 females and 20 males). Mean age of the patients was 33 years (range 3-90 years).
The initial interview for each patient was done in the hospital environment, consisting of multiple questions. Various demographic parameters such as age, cultural background, prior medical history etc. were also collected. (Table 1).
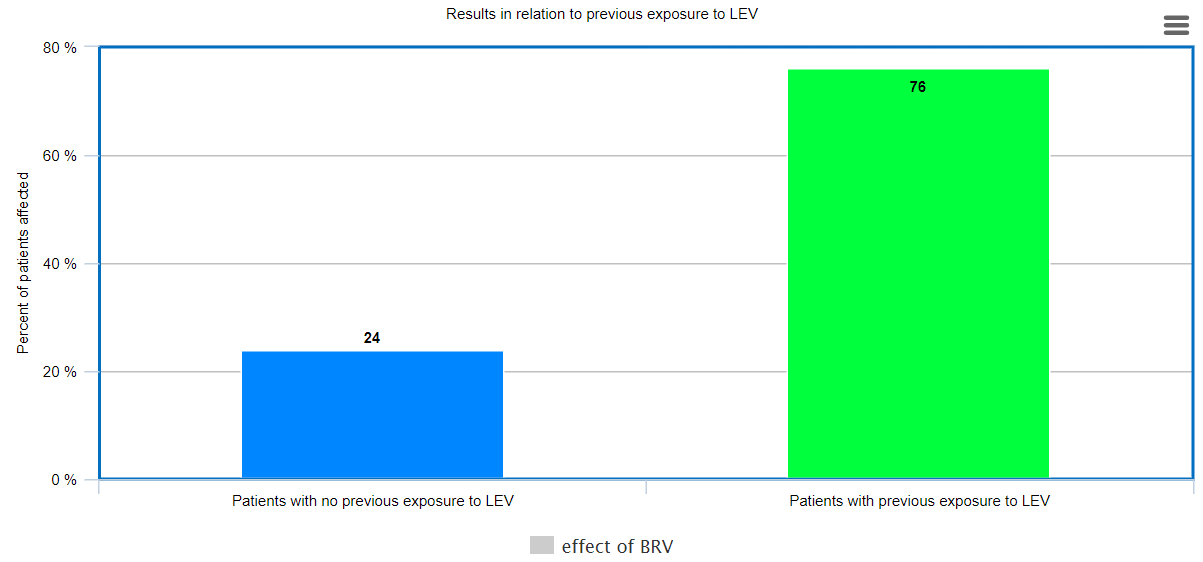
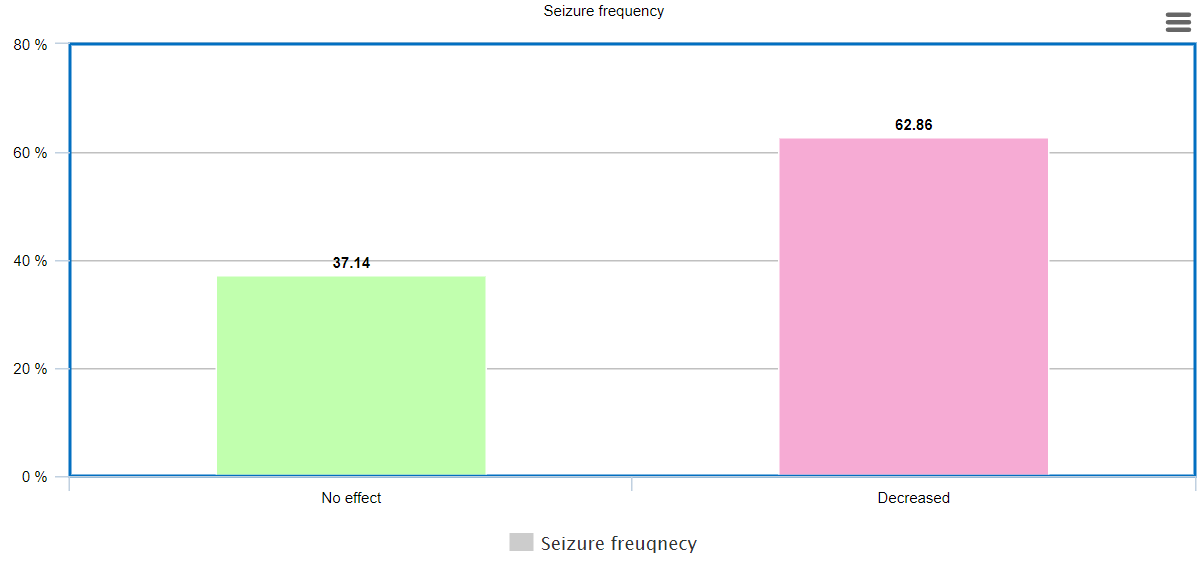
Approximately 63% patients demonstrated a decrease in seizure frequency after switching to Brivacetam. Out of these patients, 76% patients were those patients, who were earlier treated with Levetiracetam, and it was discontinued either due to inadequate control of seizures or prominent side-effects.
Table 1
ombined data of initial interview
Table 2
uantified results of the interviews conducted over the past 11-12 months
Table 3
Summary ofdependent T-test Analysis between the duration of seizure frequency (min) before and after the initiation of Brivacetam (N=35)
|
Before |
After |
t |
p |
||
|
M |
SD |
M |
SD |
|
|
|
3.5 |
2.08 |
1.57 |
0.82 |
4.48 |
0.001 |
It was also observed that Brivaracetam improves emotional balance in the treated patients (irrespective of previous exposure to Levetiracteam). Out of all the patients studied, 15 patients had emotional imbalances before and while they were on previous antiepileptics (most being on Levetiracetam). When on Brivaracetam therapy for 1 year, 12 patients felt more emotionally stable and reported decreased incidences of anger/rage, mood swings or extreme anxiety etc. Thus, it can be implied that Brivacetam has reduced incidences of emotional imbalance in patients, especially with previous exposure to LEV.
Discussion
Epilepsy affects more than 50 million people worldwide.1 Over 20 antiepileptic drugs (AEDs) are available for the treatment of epilepsy, and are approved for the treatment of several different types of seizure or syndrome. However, about a third of patients do not respond to AED treatment. 2, 3
Brivaracetam (BRV) is a new AED, approved in 2016 by the FDA for adjunctive treatment of focal seizures in patients aged ≥16 years with epilepsy. 8 BRV is a member of the racetam class of drugs, related to piracetam and the AED levetiracetam (LEV), and is the first selective ligand for SV2A.9
Previous studies have suggested that Brivaracetam reduces the seizure frequency in patients, in both treatment naïve or previously treated patients. Brivaracetam treatment also improves psycho-behavioural adverse events such as aggression and depressive symptoms associated with previous Levetiracetam treatment.13
In our study, Brivacetam was found to effectively control seizure duration and frequency in the studied patients. It was found to be effective in treatment naïve patients, and also in patients who were not adequately controlled by Levetiracteam therapy and those in whom levetiracetam was discontinued due to undesirable side effects.
Previous studies have shown that the two drugs share a common mechanism of antiepileptic action, but BRV is a high affinity synaptic vesicle protein 2A (SV2A) ligand that exceeds the binding potential of LEV by 10- to 30-fold, which could explain the high response rates, even in the patients with previous LEV exposure.”
Figure 1 showcases how patients with previous exposure to LEV had a higher percentage of reduced seizures then patients with no prior exposure to LEV, stating how 76% patients with prior LEV exposure were positively impacted with BRV (reduced seizure duration and/or frequency) whereas only 24% of patients with no prior exposure to LEV were positively affected.
hows that there is a significant difference between the mean duration of seizure episodes before (mean duration=3.5+ 2.08 mins) and after initiation of Brivacetam (mean duartion=1.57 + 0.82 mins)(p= 0.001). Thus, implying that Brivaracetam is an effective antiepileptic for seizure reduction. Similar results were found in a study Stefanatou 2021 (mentioned above). This showcases how BRV is more effective and faster in reducing seizure duration than frequency altogether. No significant relationship between the effects of Brivaracetam and gender.
Martin Hirsh et al 14 analyzed the potential for improvement of tolerability and efficacy by the use of Brivaracetam (BRV) in patients previously treated with Levetiracetam (LEV), and concluded that ntolerability or ineffectiveness of prior treatment with LEV seems not to preclude a good response to BRV. BRV was substantially better tolerated than LEV, which is similar to the results of our study.
It was also observed that Brivaracetam improves emotional balance in the treated patients (irrespective of previous exposure to Levetiracteam). Out of all the patients studied, 15 patients had emotional imbalances before and while they were on previous antiepileptics (most being on Levetiracetam). When on Brivaracetam therapy for 1 year, 12 patients felt more emotionally stable and reported decreased incidences of anger/rage, mood swings or extreme anxiety etc. Thus, it can be implied that Brivacetam has reduced incidences of emotional imbalance in patients, especially with previous exposure to LEV, similar to ealier published studies. 14
The better tolerability of BRV compared to LEV, combined with the same or better responsiveness, has been consistently reported in published clinical series in a variety of epileptic syndromes, indicating that BRV is a suitable treatment for different epilepsies.
Conclusion
To conclude, seizure control can be achieved under BRV treatment in different epileptic syndromes. BRV has a satisfactory incidence of seizure control in all seizure patients. It also has to have a good safety profile, especially in patients with previous intolerance to LEV leading to behavioral issues. BRV improves emotional imbalances found in patients with and without previous exposure to LEV. Therefore, BRV seems to be an effective, easy-to-use and safe antiepileptic drug to be used in everyday clinical practice and proves to showcase a huge scope of improvement for future prospects.