Introduction
In the last century, Neurobiologists have made significant progress in their knowledge of the active brain, which is linked to learning and memory. Using diverse methodologies, they have also clarified to some extent the linked biophysical, chemical, and biological connections inside and outside neurons. Magnetic Resonance Imaging (MRI), Magneto Encephalography (MEG), and Positron Emission Tomography (PET) are the most common techniques used to observe episodic events in the human brain under diverse healthy and pathological situations. Even yet, the precise localization of memory, how cognition and perceptions originate from the brain's stored memory loci, and how it appears in three-dimensional space, remain a mystery to this day and must be explored.1 This will necessitate a large-scale effort to develop and use innovative techniques that will allow for accurate functional mapping and modulation of neuroglial activity in the brain.2 In this case, creating a Brain Activity Map (BAM) might be a useful tool for deciphering riddles involving perception, action, personality, attitude, behaviour, memories, ideas, and awareness. The main goal of the BAM project is to develop and use new nano-tools to enable structural and functional mapping and regulation of neuroglial activity in mammalian brains.2 In reality, the goal of this study is to assess brain activity at the neuroglial circuit level, on a scale ranging from single neurons through neuron-glial function to whole-brain function.2 As a result, it will serve as a bridge for recording and manipulating the activity of whole circuits and networks in the brain, down to single-neuro-glial accuracy.
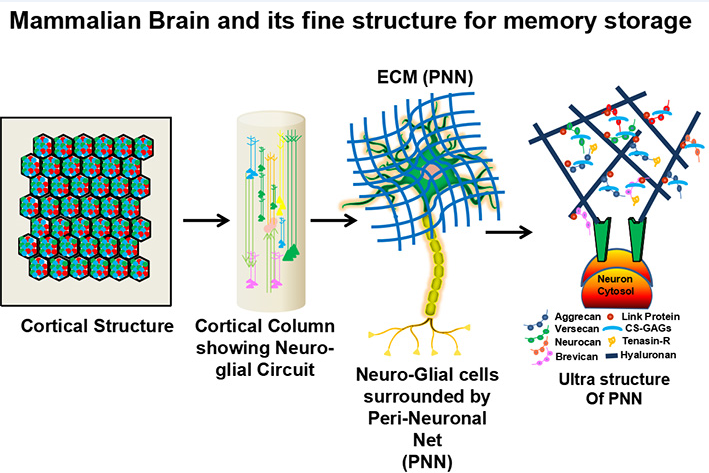
In a specialized part of the brain, an organism's memory preserves all important knowledge obtained from prior experiences. Memory can be preserved in any part of the mammalian brain, however current theories suggest that information is stored either inside neuro-glial cells via the actin cytoskeleton/micro-tubules or in the extracellular matrix (ECM) milieu, i.e. in the peri neuronal net (PNN).1, 3 The brain recapitulates earlier episodes of experiences stored at certain areas of the brain when performing any activity. For a single episode (engram) in an organism's brain, it involves billions of intricate neuro-glial connections and their trillions of tributaries (dendrites and synapses) surrounded by Extracellular Matrix (ECM). It's currently thought that ECM proteins in the Peri Neuronal Net (PNN) retain memory as information that's long-lasting, resilient, malleable, and has a lot of cross-linking with low metabolic turnover.1 Every previous ECM protein must transmit its collective stored information to the younger copies so that both the previous and new information are properly maintained, since a protein's essential characteristic travels through three separate phases: genesis, dynamics, and degeneration. As a result, despite numerous metabolic changes, information would have remained after being replicated tens to thousands of times in a long-lived organism[Figure 1]. Banerjee et al., 2017 recently discovered that PNNs, which are essential components of the ECM in the auditory cortex of adult mice, are required for fear learning consolidation.4
Endogenous proteases that digest ECM proteins, such as MMPs/disintegrin and metalloproteinase domain (ADAM), a disintegrin and a metalloprotease domain with thrombospondin motifs (ADAMTS), are now thought to be important for synaptic plasticity, dendrite/synapse formation, maturation, and relapse, and thus for associative cognitive processes.5, 6, 7, 8 All ECM proteases are localized and rapidly secreted at synapses in response to neuro-glial activity, assisting in spine enlargement/relapse required for physiological long-term potentiation (LTP) in many brain regions. 5, 6, 7, 8, 9 However, such manipulations have yet to be linked to specific behaviours or memory formation. Excitation driven by neuroglial activities has also been shown to rapidly boost MMP-9 protein expression and enzymatic activity. 6, 10, 11, 12 MMPs (MMP-9 and MMP-2) have also been shown to release outside of neuroglial cells during cerebral ischemia and to aid in neuroglial restoration following cell death in a few studies. 13 MMPs may also liberate from all regions of Neuro-glial cells and breakdown ECM proteins during cell death, neurogliogenesis, and migration.14, 15
Neuronal gene expression is thought to fluctuate dynamically in response to neuronal activity in the brain. Furthermore, out of billions of morphologically and functionally similar neuroglial cells, only a small proportion of them disseminate and encode contextual memories and specific sensory representations. As a result, a greater resolution of functional memory must be elucidated by discriminating of the individual neuro-glial subpopulations involved and their interactions in the formation of operative memory. This hypothetical article is designed to attain this objective by analyzing fear circuitry up to neuro-glia-ECM precision utilizing innovative molecular methods and exquisite imaging of complete circuits and networks in the intact brain.
The goal of the "Memory Painting" Idea is to use clarity coupled Light Sheet Microscopy to paint functional memory in the full brain inside the canvas of Neuroglia-ECM ensemble following particular behaviour in mice in three dimensional locations. I used an unique bio-molecular tool called Biologically Active Messenger (Bidirectional Messenger, BM tagged with two monomeric fluorescent proteins and Transcriptional/ Translational Messenger, TM with a single fluorescence protein) to execute this phenomena. The bicistronic promoter (cell specific plus tetracycline transactivator) will be used to express bidirectional messenger in Neuroglial cells under the control of the c-Fos tTA gene. The endogenous promoter will direct the expression of the transcriptional/ Translational messenger. All cells will be painted yellowish in hue under the steady state condition. However, during habituation, fear context, and fear context plus contextual cue, working memory will be delineated in the Neuro-glial ensemble by cell penetrating peptide guided Red, Green, and Orange colour, while the ECM will be painted by Galectin-3 guided endopeptidase mediated Far Red mesh work after cleavage by extracellular proteases at two peptide cleavage sites. After subtracting the steady state condition, the working memory for a certain behaviour will be defined as a Red Green Orange Neuroglial Ensemble surrounded by a distant far red ECM mesh work. It has not escaped my intuition that Bidirectional Messenger (Biological Boomerang) has huge implications in neuropsychiatric illnesses, generation of artificial blood, cancer therapies, and regenerative medicine in near future.
The Hypothesis
So far, much technical progress has been made in altering neural activity. 16, 17 However, the role of neuro-glial subpopulations encircled by ECM in the establishment of working memory at the circuit level has never been fully understood. In this regard, genetic modifications of activated neural ensembles or neurons involved in a sparsely stored memory engram might be a useful tool for solving the puzzle. Liu et al., 2012 used optogenetic manipulation and stimulation of hippocampus neurons to produce freezing behaviour in mice for the first time. 18 With the advent of the inducible expression of doxycycline (Dox) system, they tagged and reactivated a subpopulations of Dentate Gyrus (DG) neurons in c-fos-tTA transgenic mice by inserting optical fibre via a virus guided optogenetic vector (AAV9-TRE-ChR2-EYFP). 18 In this technique, the presence of Dox blocks c-Fos-promoter-induced tTA from binding to the cis-acting tetracycline-responsive element (TRE) site, preventing ChR2-EYFP (enhanced yellow fluorescent protein) production. In the absence of Dox, training-mediated neuronal activity labels operational c-Fos-expressing DG neurons with ChR2-EYFP, which may be reactivated during testing by induction of light. The revelation that stimulating a widespread but precise ensemble of hippocampus neurones is adequate for fear memory recall was made. In this hypothetical article, I suggest the similar dynamics of engram cell activation via c-Fos-tTA-driven Bidirectional messenger (BM) production and penetration into all neuro-glial cell populations via endogenous proteolytic activity (MMP-9) via doxycycline/tetracycline-dependent pathways.
The idea of creating BM was came from the paper of Stawarski et al., 2014, who produced a cloning-derived Forster Resonance Energy Transfer (FRET)-based biosensor that continuously displays matrix metalloproteinase 9 (MMP-9) activity. 19 This genetically encoded FRET biosensor was inserted into the cellular membrane from the outside, allowing researchers to examine MMP-9 enzymatic activity at a specific position outside the cell. 19 I propose that the BM will be expressed from all neuroglial cells and eventually anchor from the Neuroglial membrane, and that it will be cleaved and penetrate inside the same neuro-glial sup-population via protease (MMP-9) dependent cleavage with time and the advent of doxycycline/tetracycline dependent and independent pathways to conduct memory painting. The concept of fluorescent protein delivery via cell-penetrating peptide sequence (CPPS) was derived from Professor Tisens protocol20 in which his team devised a method to visualize tumour during surgery using fluorescently labeled, polycationic cell-penetrating peptide (CPP), in which released proteases from the tumour tissue cleave the linker peptide, allowing the CPP to penetrate inside the tumour cells.
Professor Tisen postulated in 2013 that very long-term memories might be retained as a pattern of holes in the Peri neuronal net (PNN), a specialized ECM that surrounds mature neurons.1 He also mentioned a genetically encoded snapshot reporter in this study, which will record the pattern of activity across a vast ensemble of neurons in a time-accurate manner.1 I suggest using galectin-3 fused endoenzyme, a carbohydrate-binding protein, to designate the PNN border of all neuro-glial sub-populations episode by episode via a protease-dependent mechanism in a tetracycline independent/dependent manner. Farhadi et al., 2018 developed a method by galectin-3 (G3) mediated endoenzyme delivery to ensure targeted delivery of an enzyme into the extracellular matrix, confirming My intuition to include this second messenger to mark the PNN boundary with far red mesh-work targeted by BM outside all neuro-glial sub populations.21
In a nutshell, I propose to "paint the fear memory engram in the mice brain encircling neuro-glia-ECM nano-domain by engineering novel chemo genetic tools (Biologically Active Messengers: BMs/ ACs plus TMs) tagged with Multi color fluorescent proteins (FP)" to illuminate the structural and functional neural ensemble for fear memory. BMs and TMs will be coloured in three distinct ways to highlight patterns of neuro-glial activity within the cells and at the ECM barrier. The cells will be yellowish in colour at control condition, but the ECM surface will take on the appearance of BMs (Far Red + Cyan/ Magenta/ Red) from two FP sources. Protease cleavage at the ECM boundary is likely to occur in the presence of an external stimulus (Fear shock/ LTP/ Light or sound cue), allowing cell specific FPs tagged with (Cyan/ Magenta/ Red) ligand to penetrate nearby cells via cell penetrating peptide (CPP), while the ECM is painted by Galectin-3 fused endoenzyme guided far reded mesh-work after cleavage by extracellular proteases at two peptide cleavage sites. As a result, at the cellular level, this change will manifest in various (Red/ Green/ Orange) RGO colours. When the same event occurs repeatedly, BMs will flow bidirectionally through particular neuro-glial cells in each episode of memory formation/elimination, resulting in a brain activity map in an RGO painted toolbox surrounded by a neuro-glia-ECM ensemble. The cleavage and penetration of FPs into neuro-glial cells by CPP and ECM via galectin-3 fused endoenzyme will proceed bidirectionally in a repeating pattern, which can be seen as "Molecular time travel" of the BMs. As a result, the Far Reded hue will be applied to a particular ECM location, resulting in a hollow mesh work of PNN proteins. A complete functional description of a brain circuit connected with a given behavioural paradigm will be represented by such a comparative comprehensive painting. This Brain Activity Map (BAM) will reveal the Functional Connectome (patterns and sequences of neuronal firing at the neuro-glial interaction level) as well as the Structural Connectome (the static anatomical map of a circuit). The comprehensive painting might be completed over several episodes and periods within a single memory. Understanding the neural codes involved in controlling a given behaviour and or mental state may be done by comparing this artwork to circuit connections and its functional or behavioural output. Such advanced painting techniques might be used to accurately diagnose and restore normal activity patterns in a brain that has been wounded or sick, paving the way for the development of larger biological and environmental applications.
Validation of hypothesis
Cloning strategy of bidirectional messenger (BM)s
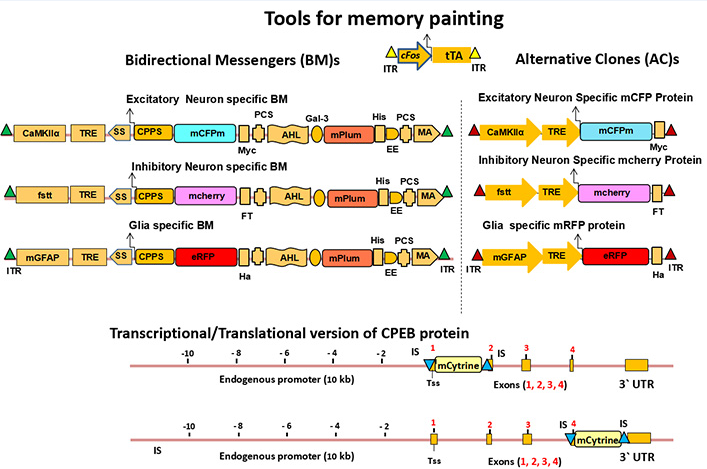
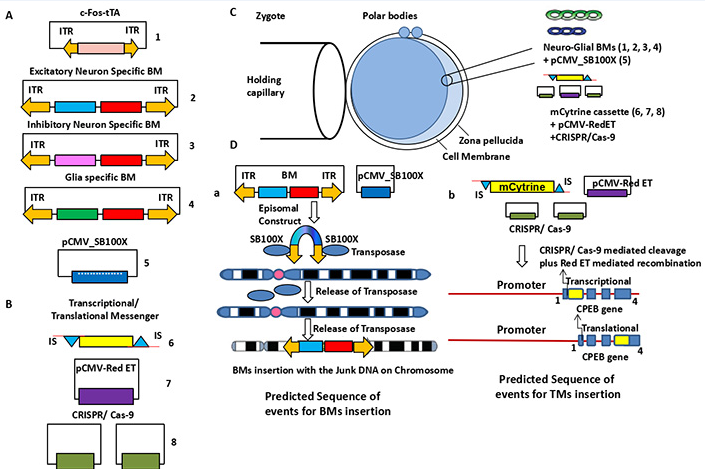
Three cell-specific BMs will be designed by tagging three monomeric fluorescent proteins with Cyan, Magenta and Red colors (CMR-FPs) (Shaner et al., 2005). Therefore after long term potentiation (LTP) in vitro / long term memory (LTM) formation in vivo, the neuroglial cells will be represented by Red, Green, and Orange (RGO) painting. 22 For this purpose, the most efficient biomolecular tool BMs will be designed with crucial domains for brain activity i.e., a Neuro-glial cell-specific promoter (CSP) coupled to a tetracycline responsive element (TRE). The promoter activity of this BMs will be regulated by tetracycline transactivator gene (tTA) under the influence of neuro-glial activity-driven promoters like c-Fos/ Arc; a secretary signal (SS); a Cell-Penetrating Peptide Sequence (CPPS) is tagged with the first XFP; 23 a Protease Cleavage Site (PCS); α helical linker (AHL) followed by linking with a Galectine 3 endoenzyme linked Far Red-FPs (Figure 2).
The BMs will be cloned using Sequence and Ligation Independent Cloning (SLIC)/ Gibson cloning methodology. 24, 25 In order to paint ECM, neuro-glial subtypes will be closed to express a fluorescent protein i.e., mPlum.22 For excitatory neurons, the BM will be designed to express the cyan tagged fluorescent protein (mCFPm) under excitatory CAMKIIα promoter and TRE. For inhibitory neurons, the BM will be designed to express the magenta tagged fluorescent protein (mCherry) under fSST promoter and TRE. For astrocyte, the BM will be designed to express enhanced Green Florescent Protein tagged (eGFP) under mGFAP promoter and TRE.26 All the three constructs will bear the sleeping beauty transposon as ITR (Inverted Terminal Repeat) element for the insertion of BMs inside the intergenic DNA.27, 28 And all the three constructs will be operated by a tTA system under strong control of c-Fos/ Arc promoter flanking sleeping beauty transposon as ITR element.27, 28 Cloning success will be checked by restriction fragment length polymorphism (RFLP) following sequencing.
Alternative Cloning strategy (AC)
Three Neuro-glia specific construct will be designed by tagging multicolored monomeric fluorescent proteins (Cyan, Magenta, Red) CMR-FPs (Shaner et al., 2005) under the regulation of Neuro-Glia specific promoter 26 plus TRE driven by c-Fos-tTA Tet-on/ Tet-off system so that after the LTP in vitro and LTM formation in vivo, the neuro-glial engram will be specified by the patterns of (Green, Red, Orange) RGO painting as per expression of this cloned BM constructs.
The CMR-XFPs will be cloned using SLIC/ Gibson cloning technology. 24, 25 For excitatory neurons, the clone will be designed to express cyan fluorescent tagged protein (mCFPm) plus Myc tag under the regulation of excitatory neuron-specific CAMKIIα promoter and TRE; For inhibitory neurons, the clone will be designed to express magenta fluorescent tagged protein (mCherry) plus FT tag under the regulation of inhibitory neuron-specific fSST promoter and TRE; For Astrocytes (glia), the clone will be designed to express enhanced Green fluorescent tagged protein (eGFP) plus Ha tag under the regulation of mGFAP and TRE promoter. As described earlier, all three constructs will bear the sleeping beauty transposon as ITR element for the purpose of insertion inside intergenic DNA on each side of the clones. 27, 28 All of the three constructs will be operated by the tTA system under strong control of c-Fos/ Arc promoter flanking sleeping beauty transposon as ITR element. 27, 28 Cloning success will be checked by restriction fragment length polymorphism (RFLP) following sequencing. 29
Recombination strategy of Transcriptional/ Translational messengers (TM)s
Cytoplasmic polyadenylation element binding protein (CPEB), is an RNA-binding protein that promotes elongation of the polyadenine tail of messenger RNA. In general, CPEB activates the target RNA for translation, but can also act as a repressor, depending on its phosphorylation state.30, 31, 32 Herein, I wish to make the Transcriptional / Translational messenger (TM) of CPEB which will tag the cells with light and deep yellow color during steady state and active state of memory formation. CPEB-XFPs will be a TM of choice because it has only four exon sequences, therefore it will be easier to engineer and it will spread everywhere inside the neuro-glial cells after long term memory formation.33
For the generation of the m-Cytrine sequence will be cloned with a 5`-3` 30 bp overhang insertion sequence (IS) complimentary to the first or last exon of the CPEB gene. The mCytrine will be inserted at first exon of CPEB gene immediately after transcription start site (Tss) as a transcriptional reporter or at last exon before the stop codon of CPEB gene as a translational reporter by pCMV-RedET mediated recombination-based technology (Gene Bridges, Germany). Before recombination the specific sequence prior to exon sequence will be cleaved by CRISPR/Cas-9.
In vitro experiments
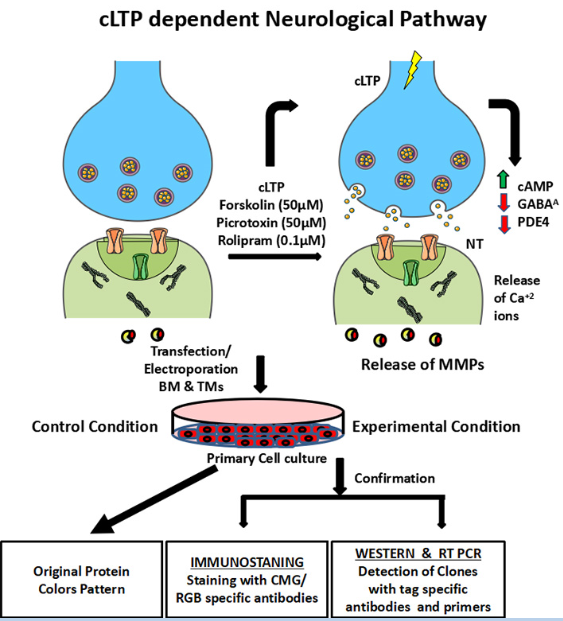
The viability and mechanism of action of BMs/ ACs and TMs will be tested in neuro-glia specific cell culture after chemically induced long term potentiation (cLTP, 50 mM forskolin, 0.1 mM rolipram, and 50 mM picrotoxin) 34. Forskolin is commonly used as a tool in biochemistry to raise levels of cyclic AMP. Rolipram is a selective phosphodiesterase-4 inhibitor discovered and developed by Schering AG as a potential antidepressant drug. Due to its interactions with the inhibitory neurotransmitter GABA, picrotoxin acts as a stimulant and convulsant. The following sequence of events is expected. (i) The cleavage of BMs and or its ACs sub-cellular localization may be checked by Western Blot with different tag-specific antibodies. Different tags will be used for two reasons. First to check whether after the cleavage the entry is occurring inside the cells and the membrane anchorage domain is remaining outside the cells and spread throughout the ECM. Secondly, during tagging with fluorescent protein if I can`t identify the fluorescent proteins then with the using of specific tags I can paint the memory engram successfully. (ii) Live cell imaging will be conducted for tracking of BMs/ ACs and TMs after cLTP applications, (iii) The molecular changes will be checked as: (a) molecular structure of BMs and TMs inside neurons, synapse and dendrites, (iv) Neuronal excitation/ inhibition will be measured by biochemical assay (Ca+2 and neurotransmitter release), ELISA, Western blots (Figure 3).
Plan of transgenic nice generation
All clones, including BMs/ACs, and c-Fos-tTA, will be inserted into the nucleus of a single cell embryo using a sleeping beauty transposon-mediated insertion technique as follows: (i) For each clone, a set of ITR sequences will be created and inserted at various locations throughout the genome sequence 35. (ii) For the generation of transcriptional/ translational variants of TM a combinatorial approach of pCMV-RedET and CRISPR-Cas-9 technology will be applied. 36 (iii) Southern blotting and PCR will be used to genotype the transgenic embryos 35, 37. (iv) Following the collection of the F0 generation, the offspring will be genotyped and segregated for behavioural testing (Figure 5).
Plan of contextual fear conditioning and fear retrieval paradigms
Many prior investigations have found memory engrams in mammalian brains, which describe the role of excitatory neurons in memory consolidation and retrieval. 18 The presence of memory engrams created by excitatory and inhibitory neurons and glia surrounded by ECM, however, has yet to be determined. In this Hypothetical Paper, I want to see if there is a memory trace (engram) formed by the Neuro-Glia-ECM border that is mirrored by "Memory painted transgenic mice" during fear training and retrieval with the contextual signal of Light/Sound. Mice will be fear conditioned (FC) for five days in context A while on diet with Doxycycline/ Tetracycline (40mg/ kg b.w.), then taken off Doxycycline/ Tetracycline for three days and fear conditioned (FC) for two days in context B. Mice will subsequently be placed back on Doxycycline/Tetracycline-containing diet and assessed in context A with fear training and light/sound stimulation for five days. Finally, in context C, Doxycycline/Tetracycline will be stopped for three days and only contextual cues (light/sound) will be delivered for two days. Animals will be acclimated to the experimental cage for 20 minutes each day in all behavioural trials. The training will take place in a sound-attenuating room with Med Associated fear conditioning rooms. Mice in the Shocked group will be placed in experimental cages and given a single foot shock (US) following a 180-second habituation interval (0.75 mA of 50-Hz pulses for 2 s). The mice will then be removed from the experiment after 60 seconds. 29
The mouse will be given 12 minutes to explore the chamber for the Light-stimulated contextual cue in Context C. The 12-minute session will be broken down into four 3-minute epochs, with the first and third epochs serving as light-off epochs and the second and fourth epochs serving as light-on epochs, respectively, after each time experiencing a foot shock as mentioned previously. 18, 29 The mouse will be exposed to light stimulation (9 mW, 20 Hz, 15 ms) throughout the whole three-minute length of the light-on episode. The mouse will be taken from the cage and returned to its home cage at the conclusion of the 12 minute period.
The mouse will be maintained in the conditioning room in context C for 500 seconds for each sound-stimulated contextual cue. At 180 s, 260 s, 340 s, and 420 s, a tone (20 s, 75 dB, 2000 Hz) will be activated, followed by a foot shock (2 s, 0.75 mA of 50 Hz pulses) 18, 29 (Figure 6).
Clarity optimized light sheet microscopy
The mammalian brain is the most complex and sophisticated organ in terms of structure; there are billions of neuro-glia cells and trillions of their tributaries, with hundreds of genetically similar cell types exhibiting a wide range of projection patterns. Clarity is a revolutionary technique that can be utilized to convert an intact brain into a hybrid form in which tissue components are removed and exogenous materials are added for greater accessibility and usefulness. (i) Following the behaviour, Doxycycline/ Tetracycline-treated and untreated brain (Home Cage Control, Non Shocked, Shocked, and Light/ Sound context) will be isolated and infused with hydro gel monomers (acrylamide, bis acrylamide, formaldehyde, and thermally triggered initiators) at 400C for 1-3 days. (ii) After day 3, the bio molecular-conjugated monomers will be thermally polymerized into a hydro gel mesh by incubating infused tissue at 370C for 3 hours. (iii) SDS micelles will be integrated next, when electric fields applied across the sample in an ionic detergent actively transport micelles into the tissue and lipids out, leaving fine structure and cross-linked biomolecules behind.38 (iv) Green, Red, Orange, Far-Red, and Yellow fluorescent antibodies will be pumped into the brain, followed by detergent-mediated antibody elimination. (v) Following three-dimensional image acquisitions using IMARIS software, the entire brain will be scanned with a Light Sheet microscope (Figure 7).
Figure 1 Memory locus in mammalian brain: Mammalian memory locus made up of billions of complex networking (Excitatory & Inhibitory Neurons and Glial cells) surrounded by trillions of tributaries (dendrites and synapses) encircled by Extracellular Matrix (ECM) for a single episode (engram). The ECM is made by Perineuronal Net (PNN). The ECM proteins in PNN perhaps store the memory as information are usually long lived, robust, plastic, having extensive cross-linkage with low metabolic turnover. 1
Figure 2 Schematics of biologically active messengers: The Bidirectional messenger (BM) will bear cell-specific promoters (Excitatory: CaMKIIα, Inhibitory: fSST, Glial: mGFAP) plus tetracycline responsive element (TRE), Signal sequence (SS), Cell Penetrating Peptide Sequence (CPPS), cell-specific variable fluorescent proteins (Cyan, Magenta and Red) CMR-FPs, Myc/ FT/ Ha tags (Excitatory/ Inhibitory/ Glial) for identification, two protease (MMP-9/MMP-2) Cleavage site (PCS), alpha helical linker (AHL), Far Red XFP (mPlum) linked with Galectine 3 fused endoenzyme, His tag, membrane anchoring (MA) domain and two inverted terminal repeat (ITR) of sleeping beauty transposon at two terminal end for insertion inside genome. The Alternative Clones (AC)s will bear the cell-specific promoters (Excitatory: CaMKIIα, Inhibitory: fSST, Glial: mGFAP) plus tetracycline responsive element (TRE), cell-specific variable (Cyan, Magenta and Red) CMR XFPs, additional Myc, FT and His tags for isolation, purification and Western Blot analysis. Both types of clones (BM and AS) will be guided by Tetracycline transactivator (tTA) under the control of neuroglial activity-driven promoters (c-Fos/ Arc) and will be controlled by the on/ off switch of Doxycycline/ Tetracyclin. The transcriptional/ translational versions of TM will carry a mCytrine at first/ last exon. The expression of TMs will be driven by endogenous promoter of the respective gene. The clones (c-Fos-tTA, and BMs/ ACs) will bear a ITR sleeping beauty transposon sequence at two complimentary end for the insertion in the genome via a pCMV-100X delivery vector. The mCytrine sequence will bear 5`-3` 30/50 base pair complimentary insertion sequence (IS) for the insertion inside first and/or last exon of the CPEB exon. This insertion will be carried out by cleavage by two CRISPR/Cas-9 vectors and pCMV-RedET Plasmid mediated recombination inside CPEB gene.
Figure 3 In vitro experiments: (i) The primary culture will be established from cortical or hippocampal origin from mice brain. (ii) c-Fos-tTA, BMs, ACs, YFP cassette, pCMV-100X, pCMV-Red-ET and two CRISPR/ Cas-9 will be introduced in the neuro-glial primary culture by lipofectamine treatment or ionotroporation. (iii) The primary neuro-glial cells will be activated by chemically induced long term potentiation (c-LTP: Forsculin, Picrotoxin and Rolipram under the regulation of on/off switch of Doxycycline. (iv) Detection of clones inside and outside of the cells after protease mediated cleavage will be with detected with tag specific antibodies through Western Blot and with specific primers by Real Time/ RT-PCR. (v)The molecular changes will be checked as follows: The expression of immediate early genes and late plasticity genes will be examined by RT/ Real time or Microarrays. (vi) Neuronal excitation/ inhibition will be measured by biochemical assay (Ca+2 and neurotransmitter release), ELISA, Western blots. (vii) Immunostaining of Memory Painting will be carried out in vitro by CMR and RGO specific antibodies.
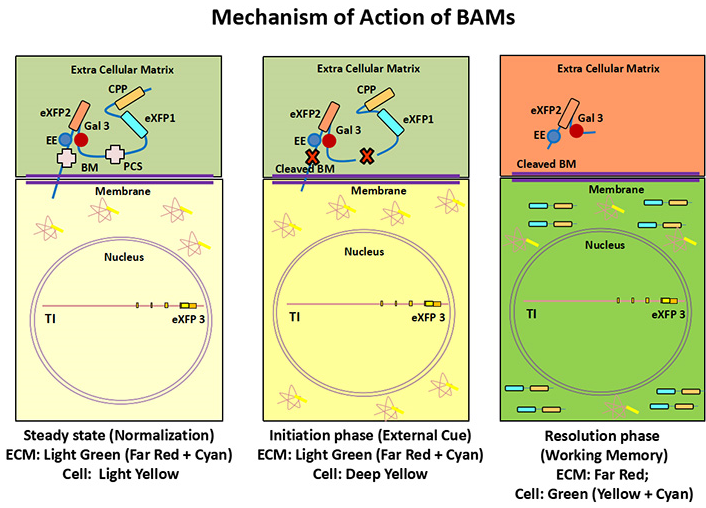
Figure 4 Mechanism of BMs and TMs inside brain: BMs and TMs will carry three different colors that will show patterns neuro-glial activity at ECM surface. At basal level (steady state condition), the cell will be yellowish however the cell surface will gain mixed color of BMs (Far Red + Cyan= green, Far Red + Magenta= Brown; Far red + Red=Red) from two FP sources. After protease cleavage CPP will penetrate inside cell with cell specific CMR-XFPs therefore yellow color will transform into different (Red/ Green/ Orange) RGO color. The specific area of memory storage (ECM) will be painted by Far Red color by Gaelctine-3 fused endoenzyme mediated delivery.
Figure 5 Generation of transgenic knock in mice: (A) BMs/ ACs and c-Fos-tTA will be introduced inside whole genome by pCMV-100X mediated transposon activity while (B) m-Cytrine will be introduced inside the first or last exon of CPEB gene by two CRISPR/ Cas-9 mediated cleavage and pCMV-RedET Plasmid mediated recombination strategy in a (C) single egg cell embryo. (D) The mechanism of introducing of BMs/ ACs and c-Fos-tTA through sleeping beauty transposon inside the genome and TMs inside the exon of CPEB gene by CRISPR/Cas-9 and Red-ET plasmid. This transgenic embryo will be introduced in a surrogate mother to produce the Transgenic “Memory Painted” Knock In mouse at F0 progeny. The construct delivery will be verified by RT-PCR by clone specific PCR primers following sequencing.
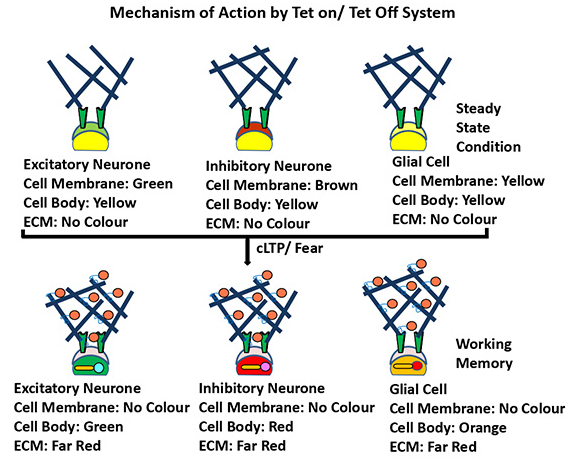
Figure 6 Mechanism of Action by Tet on/ Tet off system/Tetracycline: (A) Under the regulation of on/ off switch of Doxycycline c-Fos-tTA gene will release tetracycline that will bind the TRE element of BM/ AC constructs. Therefore CMR fluorescent proteins will be tagged with CPP sequence and far red fluorescent protein will be tagged with Galectin-3 fused endoenzyme. After the protease mediated cleavage, these CPP-CMR variants and Galectin-3-Far Red-endoenzyme variants will be targeted bidirectionally inside the neuro-glial cells and PNN respectively and transform into RGO and far red color. (B) Pictorial Representation of Memory Painting: In steady stain condition all Neuroglial cells will appear yellow color. Herein, the cell membrane (Green), cell body (Yellow) and ECM (no color) will appear inside excitatory neuron; cell membrane (Brown), cell body (Yellow) and ECM (no color) will appear inside inhibitory neuron and cell membrane (Red), cell body (Yellow) and ECM (no color) will appear inside glial cells. However, during the working memory formation after the cleavage by extracellular protease cleavage at PCS site the cell membrane (no color), cell body (Green) and ECM (far red) will appear inside excitatory neuron; cell membrane (no colour), cell body (Red) and ECM (far red) will appear inside inhibitory neuron and cell membrane (no color), cell body (Orange) and ECM (far red) will appear inside glial cells. (B) Mice will be habituated in context A with fear conditioned (FC) while on food with Doxycycline/ Tetracycline (40mg/ kg b.w.) for five consecutive days following Clarity, then taken off Doxycycline/ Tetracycline for three days and fear conditioned (FC) in context B following Clarity. Mice were then put back on Doxycycline/ Tetracycline containing food and tested for five days in context A with fear conditioning following light/ Sound stimulation following Clarity. Lastly, taken off Doxycycline/ Tetracycline for three days and only contextual cue (Light/ Sound) will be given for two days in context C following Clarity.
Figure 7 Mechanism of memory painting after clarity: Both TM and BM will give a multi-color (Red/ Green/ Orange) RGO pattern of painting in the canvas of Neuro-glial-ECM junction during formation/ elimination of memory as follows: At normalization/ Initiation phase, the ECM will appear as light green (Cyan + Red) while and cell will appear (light/ bright yellow) in color. During long term memory formation (LTM) the zone will be shifted from Cyan/ Magenta/ Red (CMR) color into mixed patterns of Red/Green/ Orange (RGO), so the excitatory neurons will appear as green, inhibitory neurons as red and astrocytes as orange in color. The ECM will appear as mesh-work of Far Red color surrounded by neuro-glial cells after memory formation with or without contextual cue/ recall. The long-term memory storage of specific engram will be demarcated as the subtracted zone of Far Red from previous event or context.
Expected Results
Generation of bidirectional messenger (BM)s/ Alternative clones (AC)s
The cloned BMs will bear all major and minor domains for cellular function. The exact location of all domains will be verified by RFLP following sequencing. Fluorescent proteins will be separated by linkers to optimize the highest fluorescent efficiency. The c-myc tag and a flag-tag will be located at the C & N terminus end to check the contribution and efficacy of BMs before and after the cleavage. The BM will be used for tracking the impression of cellular activity of a given memory engram from inside and outside of cells. Therefore, ECM and all neuro-glial membrane will seen as Far-Red in color after protease cleavage while the CPP will carry another XFP color for tagging molecular LTP inside all cells. The designing of this kind of chimeric molecule will be difficult to engineer, therefore, tagging of shorter forms of snapshot XFPs/ peptide tags (cMyc/ Flag tags) will be easier. In an alternative strategy, the desired clones may be purchased from external sources (Genscript, USA/ Gene Bridges, Germany).
Generation of transcriptional/ Translational messengers (TM)s
TMs will carry the endogenous promoter of CPEB (Figure 2, Figure 3). The endogenous promoter of TMs will direct the synthesis of either a transcriptional reporter or functional translational reporter and therefore all cells will be seen in yellow color at a basal level. As expected, after cellular activity, the other CMR-XFPs will be enter into the cells after protease cleavage, therefore, the cell will carry differential RGO painting inside the brain (Figure 4). The TMs will be useful for tracing the basal cellular and firing activities simultaneously after differential RGO painting following LTP in vitro or LTM in vivo (Figure 4). Alternatively, the desired clones may also be purchased from Industrial sources (Genscript, USA; Gene Bridges, Germany).
In vitro experiments
(i) The cleaved products of BMs bearing different FP fragments will be obtained at different sub-compartments of cells/ ECM with time after activation/ inhibition activities (ii) The live cell imaging will specify the actual time of the protease cleavage and events of LTP inside neuro-glial cells. (iii) & (iv) The anatomical and molecular details of synapse and dendrites will be obtained after LTP in neuro-glial cells. The experiment of LTP will be essential to track the inactive and active cell during neuronal firing. As the cleavage of PCS by MMP-9 will be rapid in vitro the time frame of the experiment will be narrowed down within after 10, 20, 30, 60 minutes time interval. This study will also identify the involvements of neurons and glia during LTP in vitro. As LTP/ LTD will induce/ inhibit the whole neuro-glial population, the formation of RGO patterning will be difficult to identify as it will be detected as low/ high intensity of XFP expression. Thus alternatively, the experiment will be conducted on three-dimensional neuro-glial cultures or in slice culture in vitro.
Generation of transgenic mice
The transfected embryos will be verified by southern blot and PCR analysis and transgenic mice will be collected after the F0 generation. The use of the CRISPR/ Cas-9 mediated excision and Red-ET mediated recombination strategy will minimize the cost and time for generation of transcriptional/ Translational messenger. If the sleeping beauty mediated transposition will fail, then an alternative technology may be employed by Piggyback Transposon mediated targeted delivery. In another alternative, the constructs will be ordered from the (Gen script, USA) and transgenic mice will be ordered from USA/ EU based company.
Fear conditioning and fear retrieval with contextual cue
After performing the fear conditioning experiment, prefrontal cortex, hippocampus and amygdala will be isolated; the expression of CMR proteins, early responsive and late responsive genes will be checked by Western Blot and Real-time PCR/ Microarray. Concordantly, other parameters will be studied as (a) Anatomical: The size, shape, formation, and relapsing of the dendro-synaptic projections will be checked in home cage control, non-shocked vs shocked group, Context + shocked group and fear reconsolidation/ retrieval). (b) Molecular: The molecular structure of the neurons, synapse, and dendrites are likely to be changed. After performing the fear conditioning experiment the behavioral paradigm will be plotted in context with light or sound cues with or without shock. After the fear context, more CMR proteins, early and late responsive genes are supposed to be over expressed in prefrontal cortex, hippocampus, amygdala and entorhinal cortex in comparison to home cage control or non-shocked condition. The anatomical and molecular comparison are likely to be changed as follows: (a) Anatomical: The size, shape, formation, and relapsing of the dendro-synaptic projections will be changed after fear context or in fear reconsolidation/ retrieval in comparison to home cage control and non-shocked group (b) Molecular: The molecular structure of the neurons, synapse and dendrites will be changed in case of fear context. The window of Doxycycline/ Tetracycline treatment and their withdrawal will be vital for c-Fos-tTA to become active for the occurrence of memory painting inside the mice brain. However, whether a Tamoxifen based on/off system will be an ideal and essential choice have to be identified before all experimental setup.
Image acquisition after clarity optimized light sheet microscopy (COLM)
(i) During steady state, in Tet-off system (Home Cage Control, Shocked, Non shocked, and Contextual cue) all neuro-glial cells will express the Yellow color after Clarity. (ii) In a tet-on system when Doxycycline will be withdrawn, in Fear conditioned and Contextual cue groups different subpopulations for neuro-glial cells of a fear circuit is likely to fire, therefore, in excitatory neuronal subpopulations, Cyan fluorescent protein will be expressed and mixed with transcriptional variants of CPEB-YFP (Yellow) Color and thus will appear as Green color after Clarity. (iii) Similarly, in Inhibitory neuronal subpopulations, Magenta Color will appear, mixed with CPEB-YFP (yellow) Color and will be seen as Red color after Clarity. (iv) In glial cell population, the Red color will be expressed and mixed with CPEB-YFP (Yellow) Color and will be seen as Orange color after Clarity. (v) In every individual context (Non-shocked, Shocked, Contextual cues) of working memory, firing engram of neuro-glial sub-population will decrease/ increase/ overlap/ superimpose with each other. (vi) During the long term memory formation, specific engram will be demarcated as the subtracted zone of previous and will be independent of behavioral paradigm. (Figure 7).
Discussion
The idea that memory is preserved in the brain as physical changes dates back to Plato, but it was not until the twentieth century that two driving ideas, Richard Semon's "engram theory" and Donald Hebb's "synaptic plasticity theory," were introduced. The word engram was invented by Richard Semon to explain the physical structure and operation of memory. As a result, memory consolidation refers to the transformation of a transient, labile memory into a more stable and long-lasting state 39. The memory trace or memory engram is the representation of this more stable memory in the brain, 39, 40 and the quest to understand this neurological basis of cognition has been at the forefront of neuroscience since its inception as a significant branch of research.
With the growth of human knowledge, it is becoming obvious how memories are produced, consolidated, recalled, and updated. Scientists might determine where discrete memories are produced and modified at quick time frames at either the levels of true memory or the origin of the false memory using molecular and optogenetic methods. 18, 39 A memory is thought to be encoded by a sparse population of neurons. 41, 42 As a result, such neurons may be identified and manipulated later on during learning. 43, 44, 45 Optogenetics and pharmacogenetics have now become crucial tools for identifying the neuronal populations and circuits that underpin behavior-related contextual neurological activities. 46, 47 Both methods suggest that active neural ensembles are neuronally tagged, which elucidates the neurological relevance of such neuronal ensembles in memory creation, storage, and recall.
The purpose of this hypothetical study is to present a new understanding of the molecular and cellular mechanics of memory tracing in mammals. The majority of earlier research in this area focused on manipulating memory at the excitatory/inhibitory neural level. Furthermore, as described in the current notion, firing ensembles are expected to originate continually from distinct neuro-glial subpopulations, and working memory storage may occur at the ECM border (PNN). However, this developing notion has yet to be examined in terms of memory painting methods that may be created. I propose to investigate the aforementioned phenomena using an unique "Memory Painting Idea," in which I want to map out the whole functional connectome for fear memory, as well as the related firing engrams that are responsible for fear memory and retrieval. For this, a novel "painting toolbox" is proposed to trace the entire circuitry about fear phenomenics in the canvas of the neuroglial-ECM boundary, where consciousness rarely does the intimate cohort in a harmonic and orchestrated fashion during memory reconsolidation and recall in the mammalian brain. Because this new painting concept will build a three-dimensional static memory engram throughout the full fear connectome, it will be the first of its type that can be used to work with the entire firing diagram for any specific behaviour in the mouse brain. The Brain Activity Map Project was recently initiated to record the coordinated activity of a large number of neuro-glial subpopulations. The BAM project's purpose was to find new nano devices capable of recreating the full record of neural activity throughout the entire neuronal ensemble in the mammalian brain, and eventually the human brain. 2 It is suggested here to interpret the whole record of the entire neuro-glial activity and firing engram (Brain Activity Map) for the entire connectome of fear memory related with the prefrontal cortex, entorhinal cortex, hippocampus, and amygdala in mammalian brain using the "Memory Painting" approach. 48 By tracking the patterns and sequences of whole neuroglial firing, this painting will transcend not only the "Structural Connectome" and the static anatomical map of the fear circuit, but also the dynamical mapping of the "Functional Connectome."
Neurologists have made significant advances in molecular biology tools to monitor the activity of neuronal ensembles in the mammalian brain over the last two decades. Optical approaches are less intrusive and can bring significant progress in tagging neuronal subpopulations down to single-cell precision, as well as being connected with mammalian behavior. 49 Yuste and Katz (1991)50 discovered that calcium imaging can measure the whole neuroglial activity of a circuit, allowing them to partially rebuild firing patterns of vast ensembles of neuro-glial connections in vivo. 51 A large-scale effort in neuroscience is currently underway to develop and utilize innovative nano devices that will allow functional mapping of neuro-glial activity in complete mammalian brains. This programme, as part of the BAM project, might help neurologists understand how the brain generates perception, action, memory, ideas, and consciousness, and so could be a significant step toward a full knowledge of brain function and malfunction. 2, 52, 53 This project's objective is to build a bridge that will allow researchers to record and manipulate the activity of neuro-glial circuits, and eventually the entire human brain.
I want to apply innovative tools like "Biologically Active Messengers" for Memory Painting in this hypothetical work. For painting the complete firing diagram up to neuro-glial-ECM precision, it is proposed to use one "Bidirectional Messenger" with two separate monomeric fluorescent proteins and one "Transcriptional/ Translational Messenger" with a single monomeric fluorescent protein. This might be accomplished by employing Clarity Optimized Light Sheet microscopy to trace long-term memory development following fear consolidation and recall by blending colours from CMR to RGO pattern of painting. Though I suggest using a single monomeric fluorescent protein in the Alternative approach (AC)s, it is accepted to utilize the same CMR to RGO pattern of painting in the canvas of the Neuro-glial-ECM barrier. It is suggested to employ a bicistronic promoter in both BMs and ACs, where they will be produced in all neuro-glial cells under the control of the tTA gene, which is controlled by the c-Fos/ Arc promoter. The idea of using the tTA gene to regulate the TRE via a Doxycycline/tetracycline-based on/off switch is based on the knowledge that Doxycycline is a powerful inhibitor of MMP activity. As a result, an inhibitor research using Doxycycline, Tetracycline, and Tamoxifen is required prior to the start of the project to determine which chemical will be best for the on/off switch and will not impede MMP activity. Furthermore, if alternative clones (AC) are desired for "Memory Painting," any form of on/off switch can be used because MMP activity will not be hampered. Furthermore, it is thought that distribution of fluorescently labelled proteins by CPPs into neuro-glial cells will be problematic. For the purpose of "Memory Painting," shorter variants of snapshot-FPs or different exogenous tags are recommended, as they are lengthy molecules in general. Both primary and secondary antibodies will be employed in each example, with the secondary antibodies bearing various fluorescent tags in accordance with the "memory painting" technique indicated in Figure 2.
Making transgenic mice using the standard approach described in this publication should be rather simple. All constructs, including c-Fos-tTA, BMs/ ACs, will be placed between genes (Junk DNA) using sleeping beauty induced transposition, and m-Cytrine will be inserted at the first/last exon of the CPEB gene using CRISPR/Cas-9 mediated excision and RedET mediated recombination techniques. If the above-mentioned approach fails to work, Piggyback Transposon can be employed as a backup strategy for insertion efficiency.
A major difficulty in neurobiology is obtaining high-resolution images from a complex system like the human brain while keeping its physiological viewpoint. Clarity technique was previously found for converting intact tissue into a nano-porous hydro gel-hybridized form that was optically transparent and macromolecules-permeable. 54 The authors demonstrated whole mouse brain imaging that described the morphological and anatomical aspects of long-range neuro-glial projections using mouse brains. 54 I also suggest using the same approach to map MMP-9-mediated brain activity across the complete fear connectome in order to define the firing engram of full neuro-glial cells implicated in the establishment of fear memory and recall in this hypothetical work. It is also recommended to use a far-red hue to mark the ECM border (PNN), which may be where long-term memory storage happens.1 Although this form of painting engram will interpret the full fear connectome and firing diagram in three dimensions, I also want to conduct "Memory Painting" inside the zebra fish Larvae brain in the near future, which will not require the use of a c-Fos tTA controlled on/off switch. Thus, it is suggested to paint the memory engram in four-dimensional spatiometry (with regard to time) that will be visible through the transparent brain of a zebra fish Larvae for a certain sort of behaviour. The precise phenomenon of "Molecular time travel of painting" is expected to be seen there, since the insertion of fluorescent proteins and mixing of colors by CCPs mediated transport will be continuous with individual episodes of time and will be especially reliant on the protease (MMP-9) activity. I also anticipate the utilization of specialized molecular nano-devices/materials with differential contrast that can be administered directly into the bloodstream to reveal the brain activity map mediated by protease (MMP-9) activity using a PET/MRI scanner. In the near future, the Neuro-glial receptor-attached variably coloured quantum dots with protease cleaving sites will be employed for advanced level memory painting using a PET-like scanner in fourth dimension.
Conclusion
In our last hypothetical paper, I detailed the significant accomplishments of Connectome Research. 29 Projects have previously been undertaken through the "Allen Brain Research Foundation" and "Gene Expression Atlas (GENSAT)" to better comprehend the brain's intricate circuitry at ultra structural levels. Recently, "Brain Bow" transgenic lines were developed with the purpose of generating a rainbow painting of lineage-specific neurons in the mouse brain. Finally, "Clarity" was discovered for precise structural and chemical information of a whole mouse brain with remarkable contrast of each neuroglial cell. However, no such technological breakthrough has yet been made in determining the precise site of long-term memory storage in the intact mammalian brain. In this scenario, two mega projects have recently been launched to unravel the mysteries of human intelligence and consciousness, namely the "Brain Activity Map (BAM)" project, which aims to develop new devices for mapping the entire human brain activity, and the "Blue Brain Project," which aims to decode the simulation of neuronal firing as a holographic image. The importance of the "Memory Painting" paper will have a number of ramifications in the field of neuroscience: (i) It will give a tool for determining the contribution of each excitatory versus inhibitory neuron and glial subpopulations to the formation of a specific memory with RGO pattern of painting following fear training and recall in mice. (ii) This will be the first study to look at the fear connectome, related neuroglial cells, and firing ensemble in the mouse brain during the creation and/or removal of fear memory. (iii) This will also target the PNN border, which is surrounded by neuro-glial cells and may be where extremely long-term memory is stored. 1 For the first time in the intact mouse brain, the ECM border will be delineated by Far-Reded hollow mesh work. (iv) This will invent the identified hot spots for working memory storage in the form of a rainbow pattern of painting, where neuro-glia and ECM rarely do an intimate cohort to from an engram for illuminating the molecular symphony to retrospect the earlier learning experiences episode by episode with the assumption of identical behaviour inside the mammalian brain.
Although this work will allow one to discern a static three-dimensional mapping of neuro-glial firing patterns, I hope that with the development of additional nano-devices from the BAM project, a four-dimensional mapping will be possible in the future. Because this study may simultaneously decode the underlying instructions of human brain connection and the firing engram of neurons, it may unveil the meaning of cognition and awareness, this colossal event might be a big leap for future scientific revolution. Because the neuro-glia-ECM nano-domain will be tagged with FPs of interest to unravel the fundamental connections of the mammalian brain associated with all sorts of physiological and pathological conditions, the aforementioned application-oriented research will be extremely important for the sake of the BAM project.
It also hasn't escaped my notice that "Bidirectional Messengers (BM)s [Biological Boomerang; BB]" have enormous implications and potential in generation of artificial blood and Regenerative Medicine (Neurodegenerative Disorders) to regenerate self-perpetuating Neuro-glial subpopulations in a damaged circuit via induced pluripotency or annihilation of the damaged neuro-glial cells via self-driven programmed cell death, which may have immense applications in Neurobiology and Cancer Biology to specifically eliminate the Cancer-Specific stem cells. In the near future, I expect research to begin on improving the vitality of all cells by induced pluripotency via the insertion of Yamanaka-like factors within all cells via bidirectional messengers (biological boomerangs).
Author’s Contribution
The whole idea and hypothesis was emerged to KG in the year 2014. The perception of the idea, designing and concept analysis was proposed by KG and critical analysis, manuscript writing and editing were manifested by KG.
Acknowledgments
Author acknowledges Professor Surendra Kumar Trigun and Professor Wei Jiang for their Scientific comments and minor corrections during preparation of the manuscript. Author acknowledges the Prof. Krishnendu Adhikary, HOD (MAKAUT); Assist. Prof. Shibram Bera, HOD (VU) and Director, Mr. Krishna Kanto Bhattacharjee for the recruitment of KG as faculty members of the Institute.
Statement/Ethical Approval
Consent statement/Ethical approval is required for the study for the generation of Transgenic Knock in Mice.
Abbreviations
Alternative Clone (AC); α helical linker (AHL); Brain Activity Map (BAM); Biologically Active Messengers (BAM); Bidirectional Messenger (BM); Biological Boomerang (BB); Clarity optimized Light Sheet Microscopy (COLM); chemically induced long term potentiation (cLTP); Complementary Sequence (CS); Cell Penetrating Peptide Sequence (CPPS); Cell Specific promoter (CSP); Endo Enzyme (EE); Extracellular Matrix (ECM); Fluorescent Protein (FP); Insertion Sequence (IS); Inverted terminal Repeats (ITR), Magnetic Resonance Imaging (MRI); Matrix Metalloproteinases (MMPs); Memory Painting (MP); Multi color Fluorescent Proteins (XFPs); Protease Cleavage Site (PCS); Positron Emission Tomography (PET); Peri-Neuronal Net (PNN), Secretary Signal (SS); Sequence and Ligation Independent Cloning (SLIC); Tetracycline Responsive Element (TRE); tetracycline Trans Activator (tTA); Transcriptional/Translational messengers (TM).