Introduction
Hypoglycemic encephalopathy is defined as a metabolic encephalopathy that occurs due to extremely low levels of blood glucose in the human body.1 There are many causes of hypoglycemia but Diabetes Mellitus remains the most common worldwide. It is common in both Type 1 and Type 2 forms of the disease. The hypoglycemia in Type 1 Diabetes occurs due to the reduced sympathetic responses seen in many patients while overdosage of oral hypoglycemic agents is the commonest cause in Type 2 patients.2 The presentation of the same can be both acute and chronic. Sudden onset of symptoms like fatigue, nausea, difficulty in concentration, blurring of vision and delayed responses are commonly seen.3 Focal deficits and generalized seizures can be seen in some cases. Many of the symptoms are alleviated by prompt consumption of blood glucose. In cases where the episodes are prolonged and uncorrected, they can progress to more severe presentations including lethargy, stupor, coma, vegetative state and even death.
The diagnosis of hypoglycemic encephalopathy is challenging due to poor recognition of this clinical entity and also inadequate history taking regarding the medications used and the time of onset of symptoms. The spectrum of radiological findings is diverse and non specific. It is often confused with acute ischaemic stroke due to the sudden onset of symptoms, clinical picture and imaging findings.4 Lack of prompt treatment often leads to poor long term outcomes.
We present here a case report of a seventy five year old patient with severe hypoglycemic encephalopathy with acute presentation and very poor outcome.
Case Report
This seventy five year old female patient was a known case of hypertension and Type 2 Diabetes Mellitus, on treatment with oral hypoglycemic agents and a fixed dose of insulin. She was found lying unresponsive in the bed at home at seven am in the morning. She was reported to be normal the night before and had received the night dose of long acting insulin as per her usual schedule. She was immediately shifted to the hospital and arrived in the emergency room. There, she was found to have a low blood glucose level of 46 mg/dl. She was immediately started on 25% dextrose infusion after securing vital signs. Her blood glucose levels improved to normal levels after the same. However, there was no improvement in sensorium. Central nervous system examination revealed a GCS of 3/15 with dilated and sluggishly reacting pupils. Oculocephalic reflexes were sluggish and plantars were extensor bilaterally. Motor responses to pain were absent and deep tendon reflexes were hypoactive. There were no meningeal signs. Cardiovascular and respiratory examinations were normal. Arterial blood gases showed a compensated metabolic acidosis.
In view of low sensorium, she was intubated and ventilated in the emergency room. She was later shifted to the Neuro Intensive care unit. Subsequent laboratory tests showed normal values of serum sodium, potassium, and creatinine. Lipid profiles, thyroid functions and cortisol were within normal limits. Her blood counts and acute phase reactants were normal and blood and urine cultures were sterile. Toxicology analysis of blood and urine was done which was negative. She underwent a CSF study which was normal.
In view of low initial blood glucose levels and prolonged duration of hypoglycemia, the possibility of a hypoglycemic encephalopathy was considered very likely.
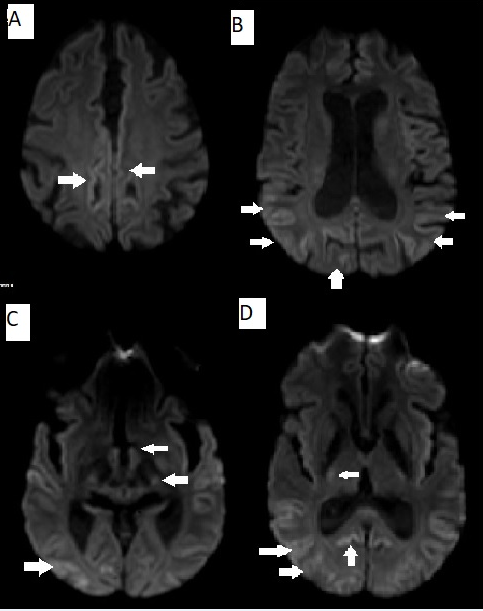
EEG done showed generalized delta wave activity suggestive of a diffuse cerebral dysfunction. She underwent an MRI of the brain (Figure 1) which showed areas of hyperintensity on T2/FLAIR sequences involving the parieto occipital cortical and subcortical areas .DWI images also showed hyperintensity in the same areas with involvement of the bilateral caudate nucleus and putamen (Figure 2). MR Angiogram done did not show any vessel obstruction. The scans were felt to be consistent with a diagnosis of a hypoglycemic encephalopathy.
She was managed with antiplatelet agents, anti convulsants glucose infusions and supportive care including physiotherapy. Even after normalization of blood glucose values, there was no improvement in patient sensorium. The overall poor outcome was explained to the patient attenders.
She was continued on treatment in the ICU. She subsequently developed aspiration pneumonia with sepsis progressing to multi organ failure. She finally succumbed to her illness after three weeks of hospital stay.
Discussion
The term hypoglycemic encephalopathy is broadly used to describe symptoms of altered sensorium as blood glucose levels fall below critical levels. Though there are no clear cut guidelines for the definition of the same, the important work of Witsch et al has defined this term as referring to a state of stupor or coma with blood glucose levels of less than 50 mg/dl and persistence of this state more than twenty four hours after normalization of blood glucose levels in the absence of other secondary factors. 5
The clinical manifestations of hypoglycemic encephalopathy are varied. They depend on the extent, speed, duration, and responsiveness of blood glucose levels in the body. The prognosis depends on many factors including the degree of hypoglycemia, duration, and the general condition of the patient with associated co morbidities.
Mild degrees of hypoglycemia cause symptoms including pale skin, recurrent sweating, hunger and associated sympathetic symptoms including palpitations, tremor and anxiety. With prolonged duration of symptoms, focal deficits including hemiplegia, aphasia, hemianopia and cortical blindness can be seen. Hypoxic injury to the basal ganglia can result in akinetic rigid states and symmetric parkinsonism. Seizures can range from generalized seizures to myoclonic jerks. Severe cases lead to decerebrate posturing, lethargy, vegetative states and coma.
The exact mechanism of tissue damage in hypoglycemic encephalopathy is not known. There are many hypothesis proposed. One of the same postulates that hypoglycemia causes a relative failure of Krebs cycle leading to production of increasing quantities of oxaloacetate from aspartic acid. This leads to tissue apoptosis and necrosis leading to widespread brain damage. A state of tissue alkalosis occurs due to raised pH and increased levels of ammonia which specifically damages neurons and tends to spare axons.6 This is in contrast to ischaemic damage that causes widespread damage of all brain tissues including glial cells and axons.
There is a very selective involvement of the nervous system in hypoglycemic damage. The parts of the brain having a very high energy consumption are affected preferentially. This includes areas like the cerebral cortex, hippocampus, cerebellum, caudate nucleus and the globus pallidus of the basal ganglia.7 Other areas include the hippocampus, subiculum, dentate nucleus and superficial layers of the cortex.
Imaging studies-especially MRI – shows features suggestive of hypoglycemic damage in most cases. Hypoglycemic encephalopathy is similar to tissue ischaemia in causing reversible cytotoxic edema mainly in the cerebral cortex and deep grey matter including the globus pallidus and thalami. White matter involvement is seen only in later stages.8 However, there is a very poor co relation between levels of blood glucose and imaging findings. Also, MRI findings do not carry relevance to long term prognosis. The main differentials for these imaging findings are acute ischaemic stroke and prion disease. The time course of the disease and the findings across multiple vascular territories may be helpful in making this differentiation.
The long term mortality rate of hypoglycemic encephalopathy ranges between 25-40 percent. Acute medical complications are the main cause of the same. Predictors of the long term outcome are limited. The initial blood glucose level and the duration of hypoglycemia have been shown to affect the prognosis. Studies have shown that a duration of hypoglycemia more than eight hours leads to a poor outcome.9 Hypoglycemia associated with Diabetes carries a worse prognosis as compared to other causes. In our case, the presence of Diabetes and the prolonged duration of hypoglycemia were both poor prognostic factors. Co-relation between MRI findings and prognosis remains poor. However, normal DWI scans on initial presentation have been shown to predict good prognosis. Presence of seizures at diagnosis has been shown to corelate with poor outcomes in some studies, but results have been variable in other trials.10 Prompt treatment and recognition of hypoglycemia have resulted in good outcomes.
Conclusion
Hypoglycemic encephalopathy is an entity that can have serious long term complications if recognized and treated late. Diagnosis is often delayed due to the varying clinical presentations. MRI imaging can help in the accurate diagnosis of this entity. Prognostic factors are still not clearly defined. More studies are needed on this topic to elucidate the pathogenesis and the factors influencing the outcomes.