- Visibility 744 Views
- Downloads 39 Downloads
- Permissions
- DOI 10.18231/j.ijn.2022.005
-
CrossMark
- Citation
Recent updates on Levetiracetam
- Author Details:
-
Salil Uppal
-
Shikhil Uppal
-
Gajanan Panchal *
Abstract
Aim: To review recent clinical evidence available for Levetiracetam in the treatment of various types of epileptic seizures.
Materials and Methods: A literature search was conducted to identify clinical studies conducted after 2015 with Levetiracetam.
Results: In patients with focal epilepsy, Levetiracetam was found to be as effective as Carbamazepine, Clobazam, and Valproic acid but with better tolerability than Carbamazepine. Levetiracetam could be used as monotherapy in the treatment of new-onset focal epilepsy. It probably has a neuroprotective benefit, particularly important in neonates and children. The safety and tolerability of Levetiracetam are more apparent during pregnancy. Thus, the adverse event profile is largely in favor of Levetiracetam in comparison to standard older AEDs. Meta-analysis has confirmed that Levetiracetam is significantly better in terms of withdrawal rates compared to the older AEDs, hence may be considered as the first line in new-onset focal epilepsy in adults and the elderly. Levetiracetam may be a better option as an add-on treatment in children with partial seizures, due to its favorable efficacy and insignificant toxicity than Oxcarbazepine and Topiramate. Recent evidence suggests that Levetiracetam could be a potential first-choice, second-line AED for Benzodiazepine resistance status epilepticus with efficacy comparable to established older AEDs. It improves the quality of life due to higher rates of seizure freedom and favorable tolerability profile.
Conclusion: Levetiracetam has the potential for being a first-line AED and has also proven to be a better adjunctive considering the recent efficacy and safety outcomes.
Introduction
Among the various neurological disorders, epilepsy is the most common chronic condition affecting neonates, adolescents, adults, and the elderly, regardless of age.[1] Globally more than 50 million people suffer from epilepsy, of which approximately 80% belong to low- and middle-income countries. Around one-sixth, approximating to 12 million of this population reside in India.[2] A recent pooled analysis of studies from 1980-2019, found the prevalence of epilepsy in India to be 4.7per 1,000 population (95% CI: 3.8–5.6).[3] The Bangalore Urban Rural Neuro-epidemiological Survey (BURNS), reported that the prevalence rate in rural communities is more than twice that of urban areas. [4] More than 57% of the total disability-adjusted life years (DALYs) in Southeast Asia due to epilepsy were accounted for from India. [5]
The incidence of epilepsy is higher in the youngest and oldest age groups with peaks within the first year and over 85 years of age.[6] However, the recent prescription analysis found maximum patients with epilepsy in the age group of 10–30 years.[7] The geriatric population is growing fastest globally and demonstrates the highest frequency of epilepsy diagnoses.[8]
As per new classification of seizures by the International League Against Epilepsy (ILAE) 2017, seizures can be classified as focal onset with a restricted area of neuronal discharge and generalized with a more diffuse neuronal discharge.[9] Focal onset seizures can be categorized as those with retained or impaired awareness. Both focal and generalized seizures are also classified as motor or non-motor onset.[9] Focal onset motor seizures include automatisms, atonic, clonic, epileptic spasms, hyperkinetic myoclonic and tonic; while non-motor include autonomic, behavior arrest, cognitive, emotional and sensory. Generalized motor seizures comprise of tonic-clonic, clonic, tonic, myoclonic, myoclonic-tonic-clonic myoclonic-atonic, atonic and epileptic spasms; while non-motor (absence) consist of typical, atypical myoclonic and eyelid myoclonia.[9]
Focal seizures are the main seizure type both in children and in adults.[6] Nevertheless, in low to middle-income countries generalized tonic-clonic seizures are more common while the incidence of status epilepticus varies from 6.8 to 41 per 100,000 per year with peaks in children younger than a year and the elderly.[6] Among the patients with epilepsy globally, nearly 30% develop refractory epilepsy, which results in a deteriorating quality of life, greater morbidity, and premature mortality.[10]
The World Health Organization (WHO) estimates that nearly 70% of the patients globally can live a seizure-free life with timely diagnosis and appropriate treatment.[1] The Antiepileptic drugs (AEDs) form the cornerstone of epilepsy treatment because it helps reduce associated morbidity and mortality, including sudden deaths. There are various classes of AEDs available for clinical use, the preference of the AED depends on the patients’ characteristics, the efficacy, and safety of the AED, including the potential for drug interactions and ease of dose titration. In the current article, we aim to review recent clinical evidence available for Levetiracetam in the treatment of various types of epileptic seizures.
Levetiracetam
Approved in 1999, Levetiracetam is a pyrrolidone compound belonging to the second-generation class of AEDs. Unlike the older AEDs, which acted via sodium or calcium channels or gamma-aminobutyric acid (GABA) receptors, newer generations inhibit GABA aminotransferase eg vigabatrin, inhibit GABA reuptake from the synaptic cleft from the synaptic cleft eg Tiagabine, modulate calcium channels (gabapentin, pregabalin), the selective non-competitive alfa-amino-3-hydroxy-5-methyl-4-isoxazolproprionic acid (AMPA) receptor antagonism eg. Perampanel, and the binding to the presynaptic SV2A receptor site eg. Levetiracetam. [11] Since SV2A protein lies on the secretory vesicle membranes mediating calcium-dependent vesicular neurotransmitter release, Levetiracetam decreases the rate of vesicle release. Apart from its affinity to SV2A, in-vitro studies in neuronal cells have shown that it competes against the activity of negative modulators of glycine- and GABA-gated currents and moderately inhibits N-type calcium channels, and acts as an AMPA receptor antagonist. [12], [13], [14], [15], [16]
It is rapidly absorbed, attaining peak plasma concentration in 60 minutes after oral administration and its bioavailability is 100%. [16] With extended-release and intravenous formulation, the time to reach peak plasma concentration is 3 hours and 5-15 minutes, respectively. [17], [18], [19] It demonstrates linear pharmacokinetics. Though food delays time to maximum concentration, its extent of absorption is unaffected, so it can be administered regardless of food. It does not compete with other drugs for protein binding sites because of minimal protein-binding (10%). The majority portion is eliminated renally unchanged, therefore dose modification is necessary for renally impaired patients and in the elderly due to decreased renal clearance.
The first-pass effect in the liver or intestine does not affect its bioavailability because Levetiracetam is minimally metabolized by oxidative enzyme systems such as cytochrome P450 (CYP450). Thus, it shows fewer pharmacokinetic interactions. No significant drug interactions occur with other AEDs Phenytoin, Valproic acid, Carbamazepine, Phenobarbitone, Primidone or digoxin, or warfarin. Its metabolism rate and clearance may be increased by other AEDs like Phenytoin, Carbamazepine, and Phenobarbitone. It may increase the central nervous system effects of centrally acting drugs. [20], [21] Since there are no major drug interactions observed, no dose adjustments are necessary with Levetiracetam in the general population, except in pregnancy due to physiological changes. [22] Since the risk of drug-drug interactions with major selective AEDs or other drugs is very low, Levetiracetam is suitable for children undergoing chemotherapy. [23]
Clinical evidence- Efficacy
Focal Epilepsy or Partial-onset epilepsy
Two network meta-analyses compared newer AEDs with Carbamazepine, which is considered the standard treatment for focal epilepsy in patients of all ages. [24], [25] Levetiracetam including other newer AEDs was considered equally effective as Carbamazepine, Clobazam, and Valproic acid, but Carbamazepine had the worst tolerability issues and associated with discontinuations in treatment naïve (new-onset) and treatment-experienced adults and the elderly with focal epilepsy.[24], [25] The Cochrane Network meta-analysis showed that for the primary outcome ‘time to the withdrawal of allocated treatment,’ for individuals with partial-onset seizures Levetiracetam performed significantly better than current first-line treatment Carbamazepine. [26] Thus, two meta-analyses suggested that Levetiracetam could be used as monotherapy in the treatment of new-onset focal epilepsy. [25]
Refractory partial-onset epilepsy
Levetiracetam has been efficacious in patients with refractory epilepsy and It shows a fairly dose-proportional percentage reduction in seizure frequency/week 12.92%, 18.00%, 11.11%, and 31.67% in the Levetiracetam 500, 1000, 2000, and 3000-mg groups, respectively. [27] When extended-release and immediate-release formulations were compared in a 12-week study in patients with refractory partial-onset epilepsy, the Levetiracetam- extended-release was found to have more than twice the seizure freedom rate over the treatment period (27.6% vs. 13.8%, respectively). [28] Moreover, the European Quality of Life-5 Dimension scores was significantly improved in the Levetiracetam- extended-release group compared to the immediate-release (7.2 vs. - 1.5, p = 0.03). [28]
Several older retrospective studies have compared the efficacy of Levetiracetam and Topiramate in the treatment of patients with refractory focal epilepsy. [29] A single-center one-year study comparing Levetiracetam and Topiramate found significantly higher retention rates with Levetiracetam at 1-year (65.6% vs 51.7%, p=0.0015) and 2-years (45.8% vs 38.3%; P=0.0046. [29] Another 2-year study found retention rate was higher in Levetiracetam users than Topiramate users.[30], [31] In a few 3-year studies specifically including patients with drug-resistant focal epilepsy, the retention rates were similar in patients using Levetiracetam or Topiramate. [32], [33] In a recent phase-IV study, Levetiracetam or Topiramate were compared as an adjunctive treatment for patients with focal seizures. The study found that the retention rate was significantly higher with Levetiracetam than Topiramate (59.1% vs 42.5%; p = 0.0086). The seizure frequency was also considerably reduced with Levetiracetam than Topiramate (74.47% vs 67.86%; p = 0.06). The difference in 50% responder rate was clinically meaningful (69.0% vs 64.8%), and the 6-month seizure-freedom rate was 35.8% vs 22.3% (p = 0.0061). [34] Not only in adults, but Levetiracetam has also demonstrated efficacy in children with uncontrolled partial-onset epilepsy. The median percentage reduction in seizures was 43.32% and the 50% response rate was 41.8%, respectively. [35] A recent phase III, 16-week trial across all ages (4-65 years) found seizure reduction in 38.7% of the participants in the Levetiracetam group, thus substantiating the findings of previous studies. [10]
A systematic review and meta-analysis including 31 studies with 1763 pediatric patients found that Levetiracetam demonstrated a higher 50% responder rate and the median percentage reduction rate. Though Lamotrigine may seem superior to Levetiracetam in terms of seizure-free rate its benefit is negated by the highest incidence of TEAE. [36] Thus, Levetiracetam is a better choice as an add-on AED in the treatment of paediatric refractory POS.
Another recent meta-analysis including 77 trials comprising 20,711 patients with refractory POS considered seizure freedom as the efficacy outcome, rather than a 50% response rate that has a little clinical relevance to epilepsy patients since the quality of life is not improved by a percentage of seizure reduction. [37] Levetiracetam was found to be equally efficacious as Topiramate, Oxcarbazepine, and Valproic acid. [37] A meta-analysis >9000 patients which considered both these parameters found that Levetiracetam-treated patients were among those with the highest odds of seizure freedom along with ezogabine and vigabatrin, while pregabalin, tiagabine, and vigabatrin had the highest odds of ≥50% reduction in refractory POS. [38]
Generalized Epilepsy
Similar to its efficacy in focal epilepsy and refractory cases, Levetiracetam also has been effective in the treatment of generalized epilepsy. Studies have demonstrated the efficacy of Levetiracetam monotherapy in juvenile myoclonic epilepsy (JME) and generalized tonic-clonic seizures alone (GTCS). An open-label study with 71.1% and 29.3% females in Levetiracetam and Valproic acid group respectively found a similar seizure freedom rate (88.9% vs 86.2%) and a withdrawal rate (8.9% vs 10.3%). [39] The time to withdrawal favoured Levetiracetam. A network meta-analysis compared AEDs with Valproic acid currently the first-line treatment in the monotherapy of generalized epileptic seizures. Levetiracetam with 47% seizure-free outcome ranked second preceded by Lamotrigine in the treatment of generalized tonic-clonic, tonic, and clonic seizures in adults and children. The therapeutic inefficacy was slightly greater with Valproic acid than Levetiracetam. [40] Thus, Levetiracetam is a useful alternative to valproate for treating generalized tonic-clonic, tonic, and clonic seizures in adults and children.
Monotherapy with Levetiracetam is considered important in genetic generalized epilepsy due to the teratogenic effects of Valproic acid particularly in women of reproductive age. The incidence of adverse events is higher in Valproic acid compared to Levetiracetam (55.1 vs 37.7%) in women of reproductive age. [39] A recent meta-analysis also concluded that Valproic acid (RR 5.82), Carbamazepine (RR 1.84), Phenytoin (RR 2.04), and Topiramate (RR 2.0) were associated with a significantly higher risk of major congenital malformations than Levetiracetam.[41] The EURAP study found that this rate was lowest with Levetiracetam (2.8%) compared to Valproic acid, Phenobarbitone, Phenytoin, Carbamazepine, Topiramate and nearly similar to Oxcarbazepine and Lamotrigine. [42] as shown in [Figure 1]. It is thus an acceptable alternative to Valproic acid particularly in women of reproductive age.
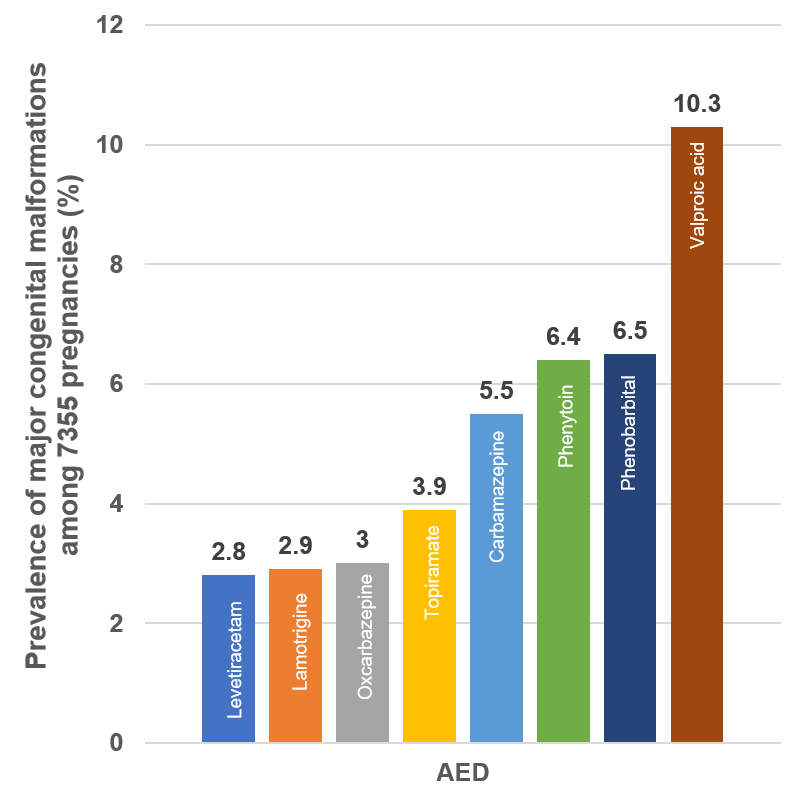
Studies with Focal and Generalized epilepsy
A 1-year RCT compared Levetiracetam with Phenobarbitone in the treatment of focal and generalized epilepsy in children (1 month- 15 years) with GTCS being the most frequent type of seizure. [43] From the initial months itself, Levetiracetam demonstrated significantly (p<0.05) better seizure remission rates than Phenobarbitone which were maintained until 9 months (3 months: 55.8% vs 44.2%; 6 months: 57.4% vs 42.6% and 9 months: 55.9% vs 44.1%). [43] Thus, Levetiracetam monotherapy was found to be more efficacious in controlling seizures in focal, generalized, and focal with secondary generalization epilepsy compared to Phenobarbitone in children.
Compared to other newer AEDs like Lamotrigine in a meta-analysis of ten studies, including 1999 elderly subjects with focal epilepsy, seizures with or without secondary generalization, it was concluded that Levetiracetam demonstrated a higher probability of seizure freedom (RR = 0.83, 95% CI = 0.68‐0.97). [44]
In patients with epilepsy refractory to two or more AEDs, Levetiracetam in combination with other newer AEDs is more effective than other AEDs alone. [45] Seizure cessation was nearly three-fold greater with Levetiracetam than without in patients treated with Perampanel (47.4% vs 15.0%; p= 0.0407). Also, the responder rate was more than three times with the combination than Perampanel alone (68.4% vs 20.0%; p=0.0076). [45]
Epilepsy expert opinion studies, from Europe and Asia in the past decade have shown an increasing preference to prescribe newer AEDs in the elderly.[46], [47], [48] The most commonly prescribed initial AED was Levetiracetam (45.5%) followed by Phenytoin (30.6%). [49]
Status epilepticus
Status epilepticus (SE) is the most frequent neurological emergency in children and the elderly leading to neurological morbidity and mortality. [50] In instances when SE is resistant to Benzodiazepines (lorazepam, midazolam, diazepam) or Benzodiazepine Refractory Status Epilepticus, intravenous Levetiracetam or Phenytoin or fosphenytoin or Valproic acid may be used as a second-line agent. The recent multicentric EcLiPSE RCT compared Levetiracetam with Phenytoin in the emergency treatment of children (6 months to <18 years of age) with convulsive status epilepticus. [51], [52] Convulsive status epilepticus ceased in a greater proportion of patients treated with Levetiracetam than Phenytoin (70.4% vs 64%). The median time to status epilepticus cessation was also lesser in Levetiracetam treated group than Phenytoin (35 min vs 45 min). [51] In another multicentric ConSEPT study, the Levetiracetam treated group had a shorter median time to seizure cessation than Phenytoin (17 min vs 22 min). [53] In a tertiary care study conducted in India comparing Levetiracetam with fosphenytoin, the seizure cessation (91.4% vs 93.1% ), seizure recurrence (17.2% vs 22.4%) rates were similar in both groups. [54] In another open-label study from India, the duration of PICU stay, hospital stay, the response latency, and seizure recurrence were similar between Levetiracetam and fosphenytoin. However, a significantly greater proportion of children received supplementary AEDs in the fosphenytoin group compared to the Levetiracetam group (31% vs 7%; p=0.0001) to control seizures. [55] In pediatric patients (3 months – 12 years) a double-blind RCT showed that control of convulsive status epilepticus (within 15 minutes) was similar in the Levetiracetam group (94%), to the Phenytoin group (89%) and Valproic acid group (83%). [56]
In a recent study, elderly patients with generalized convulsive status epilepticus were initially treated with a combination of Lorazepam with Valproic acid or Levetiracetam. In case of uncontrolled status epilepticus, the patients were crossed over to the other second-line agent from Levetiracetam to Valproic acid or vice-versa.[57] The study found that seizure control was achieved in a similar proportion of patients treated with Levetiracetam after lorazepam or Valproic acid (74.1% vs 68.3%). However, after crossing over to the second AED, Levetiracetam in combination with Lorazepam could control seizures when used as a second-line in a greater number of patients who had uncontrolled status epilepticus compared to Lorazepam plus Valproic acid (50% vs 14.2%). Other similar prospective randomized studies found seizure cessation rates of 82% vs 73.3% (adults), 92.7% vs 83.3% (children) with Levetiracetam and Phenytoin, respectively. [58], [59] IV Levetiracetam has been reported to be significantly more effective than phenytoin in children for the treatment of convulsive status epilepticus refractory to benzodiazepines.
Established Status Epilepticus Treatment Trial (ESETT) is a large RCT that compared the efficacy and safety of Valproic acid, Fosphenytoin, and Levetiracetam in patients with benzodiazepine resistant status epilepticus. The primary efficacy outcome (absence of clinically apparent seizures with improved consciousness and without additional AEDs at 1 h from the start of drug infusion) was achieved across age groups with all three AEDs namely Levetiracetam, fosphenytoin, and Valproic acid (52% vs 49% vs 52% children), (44% vs 46% vs 46% adults), and (37% vs 35% vs 47% older adults). [60] Thus, efficacy was similar with all three AEDs. [60], [61]
A meta-analysis of drugs administered for benzodiazepine-resistant status epilepticus found that Levetiracetam (69%) had similar efficacy to Phenobarbitone (74%) or valproate (76%), with better tolerability and devoid of respiratory depression and hemodynamic instability. [62], [63], [64] Another recent meta-analysis of nine studies with a total of 1732 patients showed seizure cessation occurred in 74% of patients in the Levetiracetam group and 71% in the Phenytoin group when used as second-line in patients with status epilepticus. [65] Similar to Phenobarbitone and valproate, a meta-analysis of data from seven RCTs did not find any significant difference in seizure cessation between Levetiracetam, Phenytoin, and Fosphenytoin. [66] An open-label, single-arm study found that about two-thirds (62.9%) of children had no recurrence of seizure after treatment with IV Levetiracetam. [23]
Thus, though the efficacy parameters showed no significant differences between Levetiracetam and Phenytoin, the use of the former is associated with several advantages. Owing to its broad spectrum and safer profile, it is frequently used as oral maintenance therapy for pediatric seizure control as also substantiated by the EcLIPSE study, unlike Phenytoin which is seldom used due to its non-simplified pharmacokinetics and potential toxicity. [51] Moreover, in patients on oral Phenytoin maintenance, neurologists are hesitant to use parenteral Phenytoin considering cumulative cardiotoxicity, and mortality due to arrhythmia, in contrast to intravenous Levetiracetam which raises no such safety concerns in patients on oral maintenance with Levetiracetam. Initiation of pediatric patients on oral Levetiracetam as maintenance for first-time emergency department (ED) patients with SE is more reliable due to safety and simple pharmacokinetics. An observational study in the ED setting found that only 8% commenced Fosphenytoin compared to 78% Levetiracetam as maintenance.[67] Besides the efficacy and tolerability benefits, Levetiracetam injection is also easier to reconstitute and administer compared to Phenytoin. The calculations required for reconstituting the drug, the number of vials required, and procedures needed for its administration make use of the Phenytoin complex. [52] Also Levetiracetam is available in a ready-to-use injectable formulation.
Thus, meta-analyses and available evidence in a patient suffering from status epilepticus suggest that apart from Valproate and Phenobarbitone, Levetiracetam can be used as first-line therapy in benzodiazepine-resistant status epilepticus but hints against the first-line use of phenytoin. Further, Phenobarbitone, phenytoin, and Valproate have significant safety concerns.
Clinical Evidence-Safety
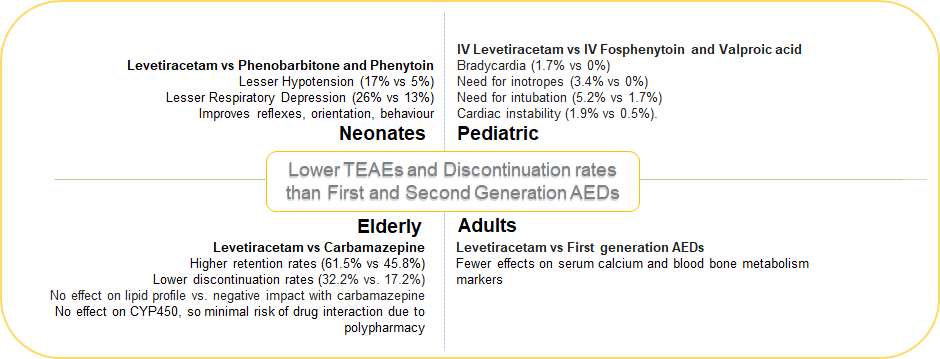
The NEOLEV2 study observed that hypotension (17% vs 5%; p<0.05), and respiratory depression (26% vs 13%) occurred in a greater proportion of neonates treated with Phenobarbitone than Levetiracetam, and vasopressor support was also required more in the Phenobarbitone group than Levetiracetam (31% vs 16%). [68] Pooled analysis of RCTs found that the risk of respiratory depression was also lesser with Levetiracetam compared to Phenytoin or fosphenytoin. [66] There were no significant laboratory or clinical abnormalities with IV Levetiracetam administration. [23]
Early exposure to Phenobarbitone used to treat neonatal seizures may negatively impact neurodevelopmental outcomes (motor, cognitive, and language performance) at 2 years of age using the Bayley Scales of Infant Development (BSID). Unlike Phenobarbitone, such cognitive and motor effects were less apparent with Levetiracetam. In a recent randomized study from Italy, the Hammersmith Neonatal Neurological Examination (HNNE) scores were significantly improved in terms of tone and posture (from 7.5 ± 1.18 to 9.36 ± 0.76; P= 0.05) with Levetiracetam while Phenobarbitone demonstrated no significant improvement (from 7.6 ± 0.96 to 8.03 ± 0.93; p=0.45).[69] Significant improvement was also noted in reflexes (from 4.86 ± 0.89 to 5.56 ± 0.49; p= 0.01) and orientation and behaviour (from 5.16 ± 1.01 to 6.7 ± 0.45; p= 0.02) with Levetiracetam while Phenobarbitone demonstrated no such significant changes. Thus, Levetiracetam with its proposed neuroprotective action and safer side-effect profile may be a suitable alternative in neonates with abnormal neurological findings. With more robust evidence being generated from the LEVNEONAT multicenter French clinical trial and NEOLEV2 trial comparing Phenobarbitone, Levetiracetam may soon be used as a first-line in the treatment of neonatal seizures. [68], [70]
Post-hoc analysis of a very recent study demonstrated higher CNS efficiency when participants were under the Levetiracetam effect. [71] In pediatric patients with both focal and generalized epilepsy, who received Levetiracetam or Phenobarbitone, Levetiracetam was well tolerated with no cognitive decline and no TEAE associated discontinuations. [43]
In short-term studies in pediatric patients with status epilepticus admitted to emergency departments, compared to IV fosphenytoin, Levetiracetam did not demonstrate bradycardia (1.7% vs 0%) or need for inotropes (3.4% vs 0%) and lesser Levetiracetam-treated patients needed intubation (5.2% vs 1.7%). [54] Similarly a large RCT comparing Levetiracetam with fosphenytoin and Valproic acid found that the Levetiracetam-treated pediatric group needed lesser endotracheal intubation than the other two AEDs (8% vs 33% vs 11%; p=0.0001). [60] Cardiac instability was also higher in the Phenytoin group compared to Levetiracetam (1.9% vs 0.5%).[65] In combination with other newer AEDs such as Perampanel, Levetiracetam had no noticeable effect on the incidence of hostility- or aggression-related AEs. [45]
The incidence of treatment-emergent adverse events (TEAEs) was also lower with Levetiracetam than Topiramate (70.6% vs 77.1%), which included somnolence, dizziness, nasopharyngitis, decreased appetite, and headache, therefore discontinuations were also lower in the Levetiracetam group than Topiramate (7.9% vs 12.7%).[34], [35] Thus, Levetiracetam has a better safety profile than Topiramate in patients with partial-onset seizures. [34] Also, in a head-to-head long-term comparison Levetiracetam has shown a higher retention rate and fewer side effects with equivalent efficacy compared with Topiramate.[29] In another 1-year safety study in patients from all age groups with refractory partial-onset seizure, Levetiracetam extended-release monotherapy demonstrated a retention rate of 65.3% and 47.1% at 12- and 18-months, with a low discontinuation rate (2.6%) and in most of the patients, mild-to-moderate TEAEs reported. [72]
Treatment of geriatric group has unique challenges, due to age-related decreased metabolism and slowed down renal clearance. This results in a reduced therapeutic window and decreased tolerability to the therapy. [73] Moreover, the probability of multiple disease conditions and consequent polypharmacy is common, which increases the likelihood of drug-drug interactions.[74] Globally, Carbamazepine, gabapentin, and Phenytoin are frequently used AEDs.[75] Nevertheless, since Carbamazepine and Phenytoin are cytochrome P450 inducers, they interact with several drug classes. [76], [77], [78] Among the newer AEDs lamotrigine, Carbamazepine and Levetiracetam have shown efficacy in the elderly. [79], [80], [81] A 58-week, multicentric RCT compared these newer AEDs in the elderly (60-95 years) with new-onset focal epilepsy. [72] At the end of the study period, the retention rate was significantly higher for Levetiracetam than for controlled release-Carbamazepine (61.5% vs. 45.8%, p = 0.02), and fairly similar to Lamotrigine (55.6%). Substantiating these findings is the observation that the patients treated with Carbamazepine experienced adverse events which led to twice the proportion of discontinuations compared to those treated with Levetiracetam (32.2% vs. 17.2%; p = 0.007), while it was intermediate for patients treated with Lamotrigine (26.3%). [82] Thus, it can be inferred that in an elderly population with exceptional treatment challenges, Levetiracetam would be a preferred alternative compared to Carbamazepine or Lamotrigine due to fewer adverse events.
Cross‐sectional studies and longitudinal repeated‐measures analyses have shown that AEDs (Carbamazepine, Phenytoin, and Phenobarbitone) which induce CYP450 enzymes also significantly increase serum lipids and C-reactive protein (CRP) in the elderly group of patients.[83], [84] A post-hoc analysis of serologic observations from a multicentric RCT over 58-weeks was performed which included elderly patients with new-onset epilepsy on monotherapy with Levetiracetam or Carbamazepine. [85] Analyses revealed that among patients not taking lipid‐lowering agents, Carbamazepine-treated patients had total cholesterol levels higher than those treated with Levetiracetam (diff 16.6 mg/dL; p=0.05). In patients on treatment with lipid-lowering drugs, the difference in total cholesterol (TC), High density lipoprotein-C (HDL-C) and low-density lipoprotein-C (LDL-C) was also greater [(TC, diff 41.4 mg/dL; p <0.0001) (HDL-C 7.22 mg/dL; p= 0.003) (LDL-C 17.59; P=0.05)]
Also, a significant drug-by-gender interaction was obvious in terms of triglyceride levels. Triglycerides were significantly higher in males taking Carbamazepine than Levetiracetam (p<0.01), while in females the difference was not significant. Among Levetiracetam-treated patients,[86], [87] Thus, in the elderly at high risk of vascular events, Carbamazepine which adversely affects its risk markers may not be a prudent choice, rather Levetiracetam would be a preferred alternative.
Several studies have found that epilepsy and older AEDs unfavourably affect the quality of semen, due to their effect on levels of sex hormones. A 6-month pre-and post-study found that Levetiracetam did not negatively affect these parameters, rather it led to a noteworthy improvement in semen quality [Pre-Levetiracetam vs Post-Levetiracetam (Semen total n x 106: 76.19 ± 30.76 to: 94.79 ± 22.06; fast forward movement rate (%): 33.64 ± 9.18 vs 39.21 ±5.81; survival rate (%) 44.27±6.38 vs 47.90 ±5.33).[88]
A network meta-analysis of 195 RCTs showed that Levetiracetam displayed the best tolerability profile compared with other AEDs, while other newer AEDs oxcarbazepine and Topiramate had a higher and lamotrigine an intermediate withdrawal rate. [88], [89] Also, a network meta-analysis in patients with refractory partial-onset seizures found safety (as measured by analysis of the withdrawal rate due to adverse effects) was poorer with Oxcarbazepine, Retigabine, and Rufinamide, whereas Levetiracetam was better tolerated. [37]
The probable mechanisms of AED-induced bone abnormalities may be related to the CYP-450 enzyme-inducing property which increases catabolism of vitamin D resulting in hypocalcemia. AEDs also may affect hormonal levels affecting bone remodeling.
Levetiracetam does not affect hepatic CYP isozymes, therefore, compared to first-generation AEDs, it has fewer effects on serum calcium and alkaline phosphatase in patients with epilepsy. [90] A meta-analysis evaluating the effect of AEDs on bone metabolism concluded that Levetiracetam may be a safer AED, compared to first-generation AEDs which decreased serum calcium, increased serum ALP more significantly, and lowered bone mineral density. [90]
From available recent evidence, Levetiracetam appears to be the most tolerated AED across the patient population with epilepsy. [Figure 2]
Summary and Conclusion
The aim of AED pharmacotherapy for epilepsy is to sustain seizure-free periods, with minimal adverse events. Thus, there lies an imminent need for AEDs which fulfill both these criteria. From the clinical evidence, we can conclude that Levetiracetam probably has a neuroprotective benefit over Phenobarbitone, particularly important in neonates since they are in process of neurodevelopment. Also, unlike older AEDs, it has no significant effect on bone metabolism, which is an important consideration in growing children and the elderly. The safety and tolerability of Levetiracetam are more apparent in reproductive issues and teratogenicity risk. [91], [92], [93]
Children exposed to Levetiracetam were not at increased risk for delayed neurodevelopment compared with unexposed children. Thus, in this patient population, the adverse event profile is largely in favor of Levetiracetam in comparison to standard older AEDs. More robust data from large studies comparing these with Levetiracetam may soon replace older AEDs at least in neonates and pediatric patients with epilepsy.
Monotherapy with Levetiracetam is more or equally efficacious to other commonly used first-line AEDs like Phenobarbitone and Carbamazepine, but meta-analysis has confirmed that Levetiracetam is significantly better in terms of withdrawal rates due to tolerability issues compared to the first-line agents, hence may be considered as the first line in new-onset focal epilepsy or POS in adults and elderly.
Adjunctive Levetiracetam is extremely beneficial in the treatment of refractory or drug-resistant epilepsy with higher or comparable seizure cessation/freedom to other newer and older AEDs due to its unique mechanism of action. Further, it has a lower incidence of TEAEs across all ages compared to Oxcarbazepine and Topiramate. Levetiracetam may be a better option as an add-on treatment in children with partial seizures, due to its favorable efficacy and insignificant toxicity. Levetiracetam also showed comparable efficacy to Valproic acid in the treatment of generalized epilepsy.
There is a large set of recent evidence which suggests that Levetiracetam can be considered as a potential first-choice, second-line AED for Benzodiazepine resistance status epilepticus with efficacy comparable to established older AEDs like Phenytoin, fosphenytoin, and Valproate or Valproic acid. It is associated with lesser morbidity compared to Phenytoin and Phenobarbitone due to the absence of cardiotoxicity, respiratory depression, arrhythmia which can increase mortality. Levetiracetam extended-release, immediate release, and parenteral formulations are thus effective in various types of epilepsy including focal and generalized epilepsy and associated
Thus, an evolving plethora of evidence confirms that Levetiracetam has emerged as an agent with the potential for being a first-line AED and has also proven to be a better adjunctive when considering the efficacy and safety outcomes. [37], [94], [95]
Conflict of Interest
The authors declare that there is no conflict of interest.
Source of Funding
Lupin Ltd., Mumbai, India
References
- . Epilepsy. . . [Google Scholar]
- Garg D. Specific considerations for epilepsy in India. Curr Med Issues. 2020;18(2):105-10. [Google Scholar] [Crossref]
- Dhiman V-, Menon GR, Kaur S. A Systematic Review and Meta-analysis of Prevalence of Epilepsy, Dementia, Headache, and Parkinson Disease in India. Neurol India. 2021;69(2):294-301. [Google Scholar] [Crossref]
- Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna D. Prevalence of neurological disorders in Bangalore, India: A community- based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23(6):261-8. [Google Scholar] [Crossref]
- Amudhan S, Gururaj G, Satishchandra P. Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol. 2015;18(3):263-77. [Google Scholar] [Crossref]
- EB. The Epidemiology of Epilepsy. Neuroepidemiology. 2020;54:185-91. [Google Scholar] [Crossref]
- Joshi R, Tripathi M, Gupta P, Gulati S, Gupta Y. Prescription pattern of antiepileptic drugs in a tertiary care center of India. Indian J Pharmacol. 2020;52(4):283-9. [Google Scholar] [Crossref]
- Hauser W, Annegers J, Kurland L. Incidence of epilepsy and unprovoked seizures in. Epilepsia. 1993;34(3):453-68. [Google Scholar] [Crossref]
- Fisher RS. The New Classification of Seizures by the International League Against Epilepsy. Curr Neurol Neurosci Rep. 2017;17(6). [Google Scholar] [Crossref]
- Manreza M, Pan T, Carbone E. Efficacy and safety of levetiracetam as adjunctive therapy for refractory focal epilepsy. Arq Neuropsiquiatr. 2021;79(4):290-8. [Google Scholar] [Crossref]
- Steinhoff BJ-, Staack AM. Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience. Ther Adv Neurol Disord. 2019;12. [Google Scholar] [Crossref]
- Niespodziany I, Klitgaard H, Margineanu DG. Levetiracetam inhibits the high-voltage-activated Ca(2+) current in pyramidal neurons of rat hippocampal slices. Neurosci Lett. 2001;306(1-2):5-8. [Google Scholar] [Crossref]
- Lukayanetz E, Shkryl V, Kostyuk P. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia. 2002;43(1):9-18. [Google Scholar] [Crossref]
- Lyseng-Williamson KA. Spotlight on levetiracetam in epilepsy. CNS Drugs. 2011;25(10):901-5. [Google Scholar] [Crossref]
- Vogl C, Mochida S, Wolff C. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol. 2012;82(2):199-208. [Google Scholar] [Crossref]
- -1 Levetiracetam. . . . [Google Scholar]
- Noyer M, Gillard M, Matagne A, Hénichart JP, Wülfert E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol. 1995;286(2):137-183. [Google Scholar]
- PP. The pharmacokinetic characteristics of levetiracetam. . 2003;25(2):123-9. [Google Scholar]
- PP. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. . 2000;85(2):77-85. [Google Scholar] [Crossref]
- Beran RG, Berkovic SF, Black AB. Efficacy and safety of levetiracetam 1000-3000 mg/day in patients with refractory partial-onset seizures: a multicenter, open-label single-arm study. Epilepsy Res. 2005;63(1):1-9. [Google Scholar] [Crossref]
- Cereghino J, Biton V, Abou-Khalil B, Dreifuss F, Gauer L, Leppik I. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55(2):236-42. [Google Scholar] [Crossref]
- . Levetiracetam 250, 500, 750 and 1000 mg tablets and 100 mg/mL concentrate for solution of infusion: summary of product characteristics. . 2010. [Google Scholar]
- Kim M, Yum M, Yeh H, Ko T, Lim H. Pharmacokinetic and Pharmacodynamic Evaluation of Intravenous Levetiracetam in Children With Epilepsy. J Clin Pharmacol. 2018;58(12):1586-96. [Google Scholar]
- Campos M, Ayres L, Morelo M, Marques FA, Pereira L. Efficacy and Tolerability of Antiepileptic Drugs in Patients with Focal Epilepsy: Systematic Review and Network Meta-analyses. Pharmacotherapy. 2016;36(12):1255-71. [Google Scholar] [Crossref]
- Lattanzi S, Zaccara G, Giovannelli F. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis. Acta Neurol Scand. 2019;139(1):33-41. [Google Scholar] [Crossref]
- Nevitt SJ, Sudell M, Weston J, Smith C, Marson A, Epilepsy C. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev. 2017;12. [Google Scholar] [Crossref]
- Inoue Y, Yagi K, Ikeda A, Sasagawa M, Ishida S, Suzuki A. Efficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizures. Psychiatry Clin Neurosci. 2015;69(10):640-8. [Google Scholar] [Crossref]
- Wu T, Lim SN, Tsai JJ, Chuang YC, Huang CW, Lin CC. A randomized, double-blind, double-dummy, multicenter trial comparing the efficacy and safety of extended- and immediate-release levetiracetam in people with partial epilepsy. Seizure. 2018;62:84-90. [Google Scholar] [Crossref]
- Bootsma H, Ricker L, Diepman L. Long-term effects of levetiracetam and topiramate in clinical practice: A head-to-head comparison. Seizure. 2008;17:19-26. [Google Scholar]
- Chung S, Wang N, Hank N. Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure. 2007;16(4):296-304. [Google Scholar] [Crossref]
- Peltola J-, Peltola M, Auvinen A, Raitanen J, Fallah M, Keränen T. Retention rates of new antiepileptic drugs in localization-related epilepsy: a single-center study. Acta Neurol Scand. 2009;119(1):55-60. [Google Scholar] [Crossref]
- Mäkinen J, Peltola J, Raitanen J, Alapirtti T, Rainesalo S. Comparative effectiveness of eight antiepileptic drugs in adults with focal refractory epilepsy: the influence of age, gender, and the sequence in which drugs were introduced onto the market. J Neurol. 2017;264(7):1345-53. [Google Scholar]
- Sunwoo J, Park B, Ahn S, Hwang S, Park C, Jun J. Three-year retention rates of levetiracetam, topiramate, and oxcarbazepine: a retrospective hospital-based study. Clin Neuropharmacol. 2017;40(2):56-62. [Google Scholar] [Crossref]
- Lee S, Lee S, Kim D. A randomized, open-label, multicenter comparative trial of levetiracetam and topiramate as adjunctive treatment for patients with focal epilepsy in Korea. Epilepsy Behav. 2019;97:67-74. [Google Scholar] [Crossref]
- Nakamura H, Osawa M, Yokoyama T, Yoshida K, Suzuki A. Effects of Long-Term Treatment with Levetiracetam as an Adjunctive Therapy in Japanese Children with Uncontrolled Partial-Onset Seizures: A Multicenter, Open-Label Study. Brain Nerve. 2015;67(11):1435-42. [Google Scholar] [Crossref]
- Cao Y, He X, Zhao L, He Y, Wang S, Zhang T. Efficacy and safety of Levetiracetam as adjunctive treatment in children with focal onset seizures: A systematic review and meta-analysis. Epilepsy Res. 2019;153:40-8. [Google Scholar]
- Hu Q, Zhang F, Teng W. Efficacy and safety of antiepileptic drugs for refractory partial-onset epilepsy: a network meta-analysis. J Neurol. 2018;265(1):1-11. [Google Scholar] [Crossref]
- Slater J, Chung S, Huynh L. Efficacy of antiepileptic drugs in the adjunctive treatment of refractory partial-onset seizures: Meta-analysis of pivotal trials. Epilepsy Res. 2018;143:120-9. [Google Scholar] [Crossref]
- Tabrizi N, Zarvani A, Rezaei P, Cheraghmakani H, Alizadeh-Navaei R. Levetiracetam in genetic generalized epilepsy: A prospective unblinded active-controlled trial. Epilepsy Res. 2019;157. [Google Scholar] [Crossref]
- Campos M, Ayres LR, Morelo M, Carizio F, Pereira L. Comparative efficacy of antiepileptic drugs for patients with generalized epileptic seizures: systematic review and network meta-analyses. Int J Clin Pharm. 2018;40(3):589-97. [Google Scholar] [Crossref]
- Andrade C. Major congenital malformations associated with exposure to antiepileptic drugs during pregnancy. J Clin Psychiatry. 2018;79(4). [Google Scholar] [Crossref]
- Tomson T, Battino D, Bonizzoni E, Craig J, DL, Perucca E. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530-8. [Google Scholar] [Crossref]
- Akter N, Rahman M, Akhter S, Fatema K. A Randomized Controlled Trial of Phenobarbitone and Levetiracetam in Childhood Epilepsy. Mymensingh Med J. 2018;27(4):776-84. [Google Scholar]
- Lezaic N, Gore G, Josephson CB, Wiebe S, Jetté N, Keezer MR. The medical treatment of epilepsy in the elderly: A systematic review and meta-analysis. Epilepsia. 2019;60(7):1325-40. [Google Scholar] [Crossref]
- Kanemura H, Sano F, Aihara M. Usefulness of perampanel with concomitant levetiracetam for patients with drug-resistant epilepsy. Eur J Paediatr Neurol. 2019;23(1):197-203. [Google Scholar] [Crossref]
- Villanueva V, Sanchez-Alvarez JC, Pena P, Puig JS, Caballero-Martinez F, AG, et al. Treatment initiation in epilepsy: an expert consensus in Spain. Epilepsy Behav. 2010;19(3):332-42. [Google Scholar] [Crossref]
- Boon P, Engelborghs S, Hauman H. Recommendations for the treatment of epilepsy in adult patients in general practice in Belgium: an update. Acta Neurol Belg. 2012;112(2):119-31. [Google Scholar] [Crossref]
- Yu P, Zhu G, Ding D. Treatment of epilepsy in adults: expert opinion in China. Epilepsy Behav. 2012;23(1):36-40. [Google Scholar] [Crossref]
- Martin RC, Faught E, Szaflarski JP. What does the U.S. Medicare administrative claims database tell us about initial antiepileptic drug treatment for older adults with new-onset epilepsy?. Epilepsia. 2017;58(4):548-57. [Google Scholar] [Crossref]
- Leppik I. Status epilepticus in the elderly. Epilepsia. 2018;59(2):140-3. [Google Scholar] [Crossref]
- Appleton RE, Rainford NE, Gamble C. Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT. Health Technol Assess. 2020;24(58):1-96. [Google Scholar] [Crossref]
- Lyttle M, Rainford N, Gamble C, Messahel S, Humphreys A, Hickey H. Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) collaborative. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (eclipse): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125-34. [Google Scholar] [Crossref]
- Dalziel SR, Borland ML, Furyk J. PREDICT research network. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393:2135-45. [Google Scholar] [Crossref]
- Handral A, Veerappa BG, Gowda VK, Shivappa SK, Benakappa N, Benakappa A. Levetiracetam versus Fosphenytoin in Pediatric Convulsive Status Epilepticus: A Randomized Controlled Trial. J Pediatr Neurosci. 2020;15(3):252-6. [Google Scholar]
- Nalisetty S, Kandasamy S, Sridharan B, Vijayakumar V, Sangaralingam T, Krishnamoorthi N. Clinical Effectiveness of Levetiracetam Compared to Fosphenytoin in the Treatment of Benzodiazepine Refractory Convulsive Status Epilepticus. Indian J Pediatr. 2020;87(7):512-9. [Google Scholar] [Crossref]
- Vignesh V, Rameshkumar R, Mahadevan S. Comparison of Phenytoin, Valproate and Levetiracetam in Pediatric Convulsive Status Epilepticus: A Randomized Double-blind Controlled Clinical Trial. Indian Pediatr. 2020;57:222-7. [Google Scholar] [Crossref]
- Nene D, Mundlamuri RC, Satishchandra P, Prathyusha PV, Nagappa M, Bindu PS. Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): A prospective randomized controlled pilot study. Seizure. 2019;65:111-7. [Google Scholar] [Crossref]
- Gujjar A, Nandhagopal R, Jacob P. Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: a prospective, randomized study. Seizure. 2017;49:8-12. [Google Scholar] [Crossref]
- Noureen N, Khan S, Khursheed A. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the management of generalized convulsive status epilepticus in children: an open-label randomized controlled trial. J Clin Neurol. 2019;15(4):468-72. [Google Scholar] [Crossref]
- Chamberlain J, Kapur J, Shinnar S. Neurological Emergencies Treatment Trials; Pediatric Emergency Care Applied Research Network investigators. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395(10231):1217-24. [Google Scholar]
- Kapur J, Elm J, Chamberlain JM, Investigators. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med. 2019;381:2103-13. [Google Scholar]
- Yasiry Z-, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure. 2014;23(3):167-74. [Google Scholar]
- Brent D, Crumrine P, Varma R. Phenobarbitone treatment and major depressive disorder in children with epilepsy. Pediatrics. 1987;80(6):909-17. [Google Scholar]
- Wright C, Downing J, Mungall D, Khan O, Williams A, Fonkem E. Clinical pharmacology and pharmacokinetics of levetiracetam. Front Neurol. 2013;4. [Google Scholar] [Crossref]
- Demott J, Slocum G, Gottlieb M, Peksa G. Levetiracetam vs. phenytoin as 2nd-line treatment for status epilepticus: A systematic review and meta-analysis. Epilepsy Behav. 2020;111. [Google Scholar] [Crossref]
- Klowak J, Hewitt M, Catenacci V, Levetiracetam. Versus Phenytoin or Fosph et al. Phenytoin for Second-Line Treatment of Pediatric Status Epilepticus: A Meta-Analysis. Pediatr Crit Care Med. 2021;22(9):480-91. [Google Scholar]
- Nakamura K, Inokuchi R, Daidoji H. Efficacy of levetiracetam versus fosphenytoin for the recurrence of seizures after status epilepticus. Medicine (Baltimore). 2017;96(25). [Google Scholar] [Crossref]
- Sharpe C-, Reiner GE, Davis SL. Levetiracetam Versus Phenobarbitone for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020;145(6). [Google Scholar] [Crossref]
- Falsaperla R, Mauceri L, Pavone P. Short-Term Neurodevelopmental Outcome in Term Neonates Treated with Phenobarbitone versus Levetiracetam: A Single-Center Experience. Behav Neurol. 2019. [Google Scholar] [Crossref]
- Favrais G, Ursino M, Mouchel C. Levetiracetam optimal dose-finding as first-line treatment for neonatal seizures occurring in the context of hypoxic-ischaemic encephalopathy (LEVNEONAT-1): study protocol of a phase II trial. BMJ Open. 2019;9(1). [Google Scholar] [Crossref]
- Gongora M, Nicoliche E, Magalhães J, Vicente R, Teixeira S, Bastos VH. Event-related potential (P300): the effects of levetiracetam in cognitive performance. Neurol Sci. 2021;42(6):2309-16. [Google Scholar]
- Chung S, Ceja H, Gawłowicz J, Mcshea C, Schiemann J, Lu S. Levetiracetam extended release for the treatment of patients with partial-onset seizures: A long-term, open-label follow-up study. Epilepsy Res. 2016;120:7-12. [Google Scholar] [Crossref]
- Brodie M, Elder A, Kwan P. Epilepsy in later life. Lancet Neurol. 2009;8(11):1019-30. [Google Scholar] [Crossref]
- Gidal BE, French JA, Grossman P. Assessment of potential drug interactions in patients with epilepsy: impact of age and sex. Neurology. 2009;72(5):419-25. [Google Scholar] [Crossref]
- Pugh M, Cott AV, Cramer J. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy. Neurology. 2008;70(22 pt 2):2171-8. [Google Scholar] [Crossref]
- Johnell K, Fastbom J. Antiepileptic drug use in community-dwelling and institutionalized elderly: a nationwide study of over 1,300,000 older people. Eur J Clin Pharmacol. 2011;67(10):1069-75. [Google Scholar] [Crossref]
- Brodie M, Mintzer S, Pack A. Enzyme induction with antiepileptic drugs: cause for concern?. Epilepsia. 2013;54(1):11-27. [Google Scholar] [Crossref]
- Vecht C, Wagner G, Wilms E. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2(7):404-9. [Google Scholar] [Crossref]
- Brodie MJ, Overstall PW, Multicentre GL. double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res. 1999;37(1):81-7. [Google Scholar] [Crossref]
- Rowan A, Ramsay R, Collins J. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868-73. [Google Scholar] [Crossref]
- Saetre E, Perucca E, Isojarvi J. An international multicenter randomized double-blind controlled trial of lamotrigine and sustainedrelease carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia. 2007;48(7):1292-302. [Google Scholar]
- Werhahn K, Trinka E, Dobesberger J. A randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsy. Epilepsia. 2015;56(3):450-9. [Google Scholar] [Crossref]
- Eiris J, Novo-Rodriguez MI, Rio MD, . The effects on lipid and apolipoprotein serum levels of long-term carbamazepine, valproic acid and Phenobarbitone therapy in children with epilepsy. Epilepsy Res. 2000;41(1):1-7. [Google Scholar] [Crossref]
- Nikolaos T, Stylianos G, Chryssoula N, Irini P, Christos M, Dimitrios T. The effect of longterm antiepileptic treatment on serum cholesterol (TC, HDL, LDL) and triglyceride levels in adult epileptic patients on monotherapy. Med Sci Monit. 2004;10(4):50-2. [Google Scholar]
- Mintzer S, Trinka E, Kraemer G, Chervoneva I, Werhahn KJ. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid-lowering agents in the elderly. Epilepsia. 2018;59(10):1899-907. [Google Scholar] [Crossref]
- Ucar M, Neuvonen M, Luurila H. Carbamazepine markedly reduces serum concentrations of simvastatin and simvastatin acid. Eur J Clin Pharmacol. 2004;59(12):879-82. [Google Scholar] [Crossref]
- Bullman J, Nicholls A, Landingham KV, Fleck, R, Vuong A, Miller J. Effects of lamotrigine and phenytoin on the pharmacokinetics of atorvastatin in healthy volunteers. Epilepsia. 2011;52(7):1351-8. [Google Scholar] [Crossref]
- Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol. 2017;73(7):811-7. [Google Scholar] [Crossref]
- Wu D, LC, Ji F, YS, Sun H. The effects of oxcarbazepine, levetiracetam, and lamotrigine on semen quality, sexual function, and sex hormones in male adults with epilepsy. Epilepsia. 2018;59(7):1344-50. [Google Scholar] [Crossref]
- Fu J, Peng L, Li J, Tao T, Chen Y. Effects of Second-Generation Antiepileptic Drugs Compared to First-Generation Antiepileptic Drugs on Bone Metabolism in Patients with Epilepsy: A Meta-Analysis. Horm Metab Res. 2019;51(8):511-21. [Google Scholar] [Crossref]
- Chowdhury A, Brodie MJ. Pharmacological outcomes in juvenile myoclonic epilepsy: support for sodium valproate. Epilepsy Res. 2016;119:62-6. [Google Scholar] [Crossref]
- Grünewald R. Levetiracetam in the treatment of idiopathic generalized epilepsies. Epilepsia. 2005;46(9):154-60. [Google Scholar] [Crossref]
- Kowski A, Weissinger F, Gaus V, Fidzinski P, Losch F, Holtkamp M. Specific adverse effects of antiepileptic drugs-A true-to-life monotherapy study. Epilepsy Behav. 2016;54:150-7. [Google Scholar] [Crossref]
- Zhuo C, Jiang R, Li G. Efficacy and Tolerability of Second and Third Generation Anti-epileptic Drugs in Refractory Epilepsy: A Network Meta. Analysis. Sci Rep. 2017;7(1). [Google Scholar] [Crossref]
- Yi Z, Wen C, Cai T, LX, ZX, ZS. Levetiracetam for epilepsy: an evidence map of efficacy, safety and economic profiles. Neuropsychiatr Dis Treat. 2018;15:1-19. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Uppal S, Uppal S, Panchal G. Recent updates on Levetiracetam [Internet]. IP Indian J Neurosci. 2022 [cited 2025 Sep 29];8(1):21-30. Available from: https://doi.org/10.18231/j.ijn.2022.005
APA
Uppal, S., Uppal, S., Panchal, G. (2022). Recent updates on Levetiracetam. IP Indian J Neurosci, 8(1), 21-30. https://doi.org/10.18231/j.ijn.2022.005
MLA
Uppal, Salil, Uppal, Shikhil, Panchal, Gajanan. "Recent updates on Levetiracetam." IP Indian J Neurosci, vol. 8, no. 1, 2022, pp. 21-30. https://doi.org/10.18231/j.ijn.2022.005
Chicago
Uppal, S., Uppal, S., Panchal, G.. "Recent updates on Levetiracetam." IP Indian J Neurosci 8, no. 1 (2022): 21-30. https://doi.org/10.18231/j.ijn.2022.005
