Introduction
Subthalamic nucleus (STN) deep brain stimulation (DBS) is an effective therapeutic treatment for the motor symptoms of Parkinson disease or Parkinson`s disease (PD).1 The D B S entails the and necessitates the embedding surgically of a quadri - polar microelectrode into the motoric territory of S T N, followed by constant induced stimulations through the linking of the electrode to a surgically implanted pulse generator (I P Gs). An important factor contributing to the efficiency of DBS is the precise point of the sub thalamic nucleus in the human brain, in particular PD brain. The tiniest volume of the STN motor territory, its depth from the cortical surface, and its proximity to other critical neural structures, make precise targeting crucial as well as challenging. 2 Together with stereotactic imaging, intra operative micro electrode recording (M E R) system is the most commonly used physiological technique to determine the S T N site for constant implantation of DBS macro electrodes.3 In micro electrode recording, single neuron activity (SNA), i.e., the electrical activity resulting from individual neurons, is recorded through micro electrodes characterized by a modest or a small-scale diameter and a high impedance, electrical resistance. The resulting signal patterns are interpreted to confine the anatomical borders of S T N.4 The initial trajectory and stereotactic coordinates of the target are determined based on preoperative either T1 weighted magnetic or functional magnetic imaging (M RI or f-MRI) and/or computed axial tomography (C A T) images. Then the micro electrodes are placed through cannulas into the brain and SNAs are acquired. 5 The number of MER trajectories being used in localization can vary based on technical factors and institutional preference from one to five or more. 1 Following the M E R target localization, microelectrodes are withdrawn and replaced by the quadripolar DBS macroelectrode. Although MER provides useful information for guiding surgery, the procedure carries a risk of intracranial hemorrhage due to usage of multiple electrodes and sharp tip of these microelectrodes. 6 Moreover, the interpretation of signal characteristics by neurophysiologists or neurosurgeons makes the procedure more open to human error with the increased surgical time, especially in the multi-target cases requiring MER interpretation that is more complex. 4, 5, 6, 7, 8, 9
Unlike MER-SNA, macro electrode recordings are based on local field potentials which represent the aggregate activity of neuronal populations in the region of the electrode contact. 7, 8, 9, 10 In PD, LFP recordings from STN are an important indicator of neural rhythms. 8 Studies have demonstrated an excessive synchrony in beta band (13Hz-30Hz) activity in STN. 9, 10, 11, 12, 13, 14, 15
The aim of the present study was to explore the informational content of local field potential’s L F Ps recorded from macro-D B S electrodes, in order to identify the anatomical borders of S T N. Since the L F Ps can easily be recorded from macro contacts, their use in the operating room can reduce surgery time and serve as a useful tool for target confirmation or validation.
Aims and Objectives
To investigate whether field potentials activity can be employed for subthalamic nucleus (STN) edge or perimeter detection. The subthalamic nuclei STN perimeter detections by means of local field potentials L F Ps were evaluated to border or edge predictions by way of the functional stereotactic DBS neuro surgeon, based on micro electrode derived, single unit recordings (M E R – S N A of subthalamic nucleus S T Ns).
Materials and Methods
The subjects and functional DBS-surgery
Twenty six patients provided informed consent and with approval of the institute ethical committee were participated in this study. All study subjects had a diagnosis of advanced i d i o p a t h i c P D and exhibited typical Parkinsonian motor symptoms despite optimal medial therapy. All patients discontinued Parkinson’s medications 12 hours prior to surgery. Per standard clinical routine in our institution, three simultaneous M E R – S N A signals recording tracks were executed in every subject using local anesthetic alone. The initial target and trajectory were identified by stereotactic M R I fused together to a stereotactic computed axial tomography (the CAT) on a neuro navigational platform of Medtronic. Microelectrode implantation and asynchronous (parallelly and/or concurrently) S N A signal acquisitional recordings were obtained by employing a N e u r o d r i v e and Micro guide system accordingly. Following M E R – S N A, all the patients underwent bilateral implantation of the deep brain stimulation D B S microelectrodes into the subthalamic nucleus S T N (D B S e l e c t r o d e m o d e l number 3389 of Medtronic. These D B S microelectrodes include f o u r p l a t i n u m–iridiu m c y lin d ric a l surfaces from deepest contact 0 to most superficial contact 3 (1.27 mm diameter and 1.5 mm length) and a center-to-center distance of 2 mm.
Subthalamic nucleus local field signals acquisitions
A retrospective study was carried out at a tertiary care hospital with a dedicated movement disorder unit from South India. 46 patients with diagnosis of PD as per United Kingdom Parkinson disease society brain bank criteria were included. All the patients were willing to undergo the procedure and fulfilled the following criteria to be eligible for STN-DBS i.e., they had disease duration of 6 years or more, good response to tuned regimen of oral Levodopa (L-dopa, the metabolic precursor of dopamine), able to walk independently in drug “ON” state and had normal cognition. All PD patients who were wheelchair or bed bound, had dementia or severe psychiatric disturbances were excluded.
Surgery was performed in all by a qualified neurosurgeon. Stereotactic targets were acquired using a specialised system with a stereotactic frame (CRW) which has a luminant MR localiser. The targeting was performed according to Lozano’s technique – 2mm sections are taken parallel to the plane of anterior comissure-posterior commissure line and at the level with maximum volume of red nucleus, STN is targeted at 3 mm lateral to the antereo-lateral border of red nucleus.
The co-ordinates are entered into stereo-calc software which gives the co-ordinates of the STN. Another neuro navigation software –Framelink is also used to plot the course of the electrodes and to avoid vessels. The surgery is performed with two burr holes on the two sides based on the co-ordinates. 5 channels are introduced with the central channel (as channel 1) representing the MRI target while medial (nearer the centre) representing as channel 2 and lateral (away from the centre) representing as channel 3 are placed in the x axis while anterior(front) representing as channel 4 and posterior (back) representing as channel 5 are placed in the y axis to cover an area of 5 mm diameter.
Intra-operative recording was performed in all 5 channels. All five microelectrodes are slowly passed through the STN and recording is performed from 10mm above to 10mm below the STN calculated on the MRI. STN IS identified by a high noise with a large baseline and an irregular discharge with multiple frequencies. Figure 2 shows the microelectrode recording which is obtained from the STN The channel with maximum recording and the earliest recording were recorded on both sides. Intra operative test stimulation was performed in all channels from the level at the onset of MER recording. Stimulation was done at 1mv, 3mv to assess the improvement in Bradykinesia (akinesia), rigidity and tremor. Appearance of dyskinesias (side-effects) was considered to be associated with accurate targeting. Side effects were assessed at 5mv and 7mv to ensure that the final channel chosen had maximum improvement with least side effects.
Correlation was assessed between the aspects of MER and the final channel chosen in 26 patients (92 sides). STN LFPs were acquired. The initial monopolar LFP recording generally started 20 mm above the estimated target and continued until the electrode reveryed -3 mm below microelectrode M E R determined target. The N e u r o d r i v e was used to drive the electrode down to the estimated target using 1 mm steps until 10 mm above estimated target and then the step size was reduced to 0.5m m. The L F P data were acquired from all four contacts of the deep brain stimulation D B S microelectrode along with E.K.G signal for thirty30seconds duration at every depth.
The acquired signals were sampled at 2kHz with a 16-bit A/D (2N = 216, N = 65536 sample data points) dynamic-resolutions. All raw data channels were high pass filtered at 0.1 Hz. Signals were transferred into a PC for MatLab(off-line) spectral/power-spectral-analysis.
Judgments
Recorded LFP data were marked and graphically-visualized followed by the export to the mathematical software tool box i.e., technical computing Mat-Lab software with statistical tool boxes for processing. The local field potential L F P data from all four contacts were filtered with low-pass filters employing an finite impulse response filter with a 512Hz cutoff frequency, and then down-sampled to 1000 Hz for the signal-analysis purposes.8 During preprocessing of the field potential’s data i.e., L F P data, mono-polar signals were converted into bi-polar derivations:0 to 1,1 to 2, 2 to 3. It ought to be observed that every bi-polar contact represents the L F P activity at different depths with 2 mm space. Subsequently, the L F P data derived from all bi-polar contacts that sample various depths were mixed and processed simultaneously. In order to explore the frequency content of the L F P data at every depth, we generated a depth-frequency analysis similar to a time-frequency analysis. We observed that the LFP data were corrupted by many factors including tremor and/or environmental factors in the operating room setting. Therefore, we computed the LFP spectrum with a modified Welch periodogram method, including robust statistics. Simply, rather than using an average over different segments, we used a median operator to compute the periodogram which suppressed outliers. Specifically, for spectrum analysis, the fast Fourier transform (FFT) was computed with a 512 samples long Hamming window (to minimize the spectra of the signal) and the window was shifted with 50 % overlap. After computing the squared magnitude in every sliding window, we used the median operator to estimate the LFP spectrum. We repeated this same procedure at every depth and the resulting spectra were used to visualize dynamic frequency spectrum of the LFP data.
Post-Processing
We investigated the depth-frequency maps and extracted the energy of LFP sub-bands at every depth to identify the superior STN border. The sub-band energy values at every depth were first filtered by zero-phase filtering in both forward and reverse directions to smooth the data. Then, output was interpolated with 0.5 mm resolution. Instead of joining data points by straight line segments using a linear interpolation, a cubic interpolation method was chosen. Finally, the interpolated signal was normalized between zero and one with a Max-Min method.
For identifying the superior subthalamic nucleus S T N border using normalized sub-band energy features, we first determined a 10% threshold to find noticeable energy increase with respect to higher depth values. Then, we computed the first derivative of the data to inspect the change in energy of consecutive data points. We identified the superior STN border using the following criteria:
Energy value exceeds the 10% threshold
The slope of the signal is positive for the three consecutive points
The slope was taken into account after 7 mm and below. I in order to compare the borders identified by MER-SNA and LFP, a paired student t-test was conducted. Moreover, the root mean square (RMS) of these differences was calculated.
Results
The fresh data of local field potentials of a characteristic Parkinson subject is displayed in (Figure 1a). Archetypal aliasing ripples that are resulted from sudden unexpected unforeseen movements of the PD-patient and supplementary conservational factors can be viewed at greater depths. Following the electrode, valued a convinced and positive depth, higher amplitudes of local electrical field potential activity was examined. The transient of this amplitude transpired dependably or reliably in all PD-subjects amongst the greater and mediocre boundary of sub thalamic nuclei as detected by the M E R – S N A. To yield an aroma or additive piquancy about the frequency content of local field electrical potential activity at various depths, the depth-frequency atlas of the identical PD-subject is illustrated in (Figure 1b). We perceived a vibrant progress in the
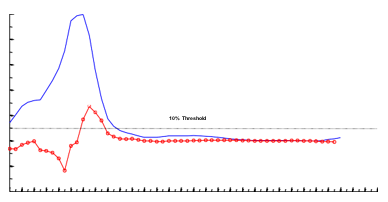
In Figure 2, the sub band energy stratagems for all PD-subjects in the β-bands and γ-bands are established. The sub band data of all the PD-subjects were normalized to its maximum value and aligned with respect to the average superior border of the nuclei-i.e., S.T.N, see red dashed-line. Excluding one PD-subject, see the data in the orange color depicted in all cases the β-band energy is well connected through the S T N greater boundary. The Figure 3 gives the inconsistency values of characteristic PD-subject by the alterations of successive data points at every depth at β-band. In order to select the superior border of S T N, a 10 % threshold was applied and the first data point below 7milli meters passing the threshold and having a positive slope, i.e., increasing energy-trend, was nominated as the greater boundary. Equal to 7 mm, all the PD-subjects were having a dependable and reliable alteration and 7milli meters was the first-point having an amplified augmented normal deviation, see the flushed-pinky shaded-region from the mediocre and the improbable likelihood of above depths being the highest-perimeter, i.e.,10mm is conforming 7mm above the normal larger limit), 7mm was selected as threshold depth-significance.
The mean-value of greater-s t n perimeter expected through m e r s was 3.62±0.93m m while the mean-value of larger-boundary derived from macro-electrode l f p signal acquisitions was 4.68±1.04m m and 4.09 ±1.57m m in β and
Figure 1
Clockwise (a) Samples vs. Depths (mm). The STN neurons activity at various levels (−2 to +5 mm). Here, -Ve values correspond with positions above the STN-target. The exterior part of STN: −1mm to 0mm is visibly identified by enhance in milieu-noise and an abrupt enhance in liberation charge distinguished by periodic explode movement (15Hz-25Hz). Cavernous layers of STN-neurons (+1.5mm to 3.5 mm) depict new uneven above the baseline frequency liberation-pattern. L.F.P raw-data of a characteristic PD-subject though the electrode moved from 21mm above the target down to -4.5mm underneath it. (b) Depth (mm) vs. frequency (Hz).spectral analysis for the characteristic-pd-subject. High-pass filtered at2.5Hz. Black arrows show the greater and inferior-S T N boundaries, correspondingly from left-hand to correct. (c) Samples vs. depth (mm) Bi polar-L F Ps band-passed, filtered at 13Hz-30Hz for the demonstrative-PD-subject. (d) Samples vs. depth (mm). The MER signal patterns of STN neurons for various-depths: -4mm to +10mm. It can be observed that the thin part of the STN is recognized by amplifying background-noise and a rise in discharge pace differentiated by the rhythmic-bursts-of-activity with higher-frequencies (neurons 3, 4, 5 and 6). The negative (-4 to -1)) values correspond with positions above the MRI-based target. Deeper layers of the STN show a more irregular-high-frequency discharge-patterns. Bi polar L F Ps high-pass filtered at 48Hertz. Bloodshot enflamed or inflamed-red dashed-lines demonstration the higher and lowest perimeters of the nuclei.
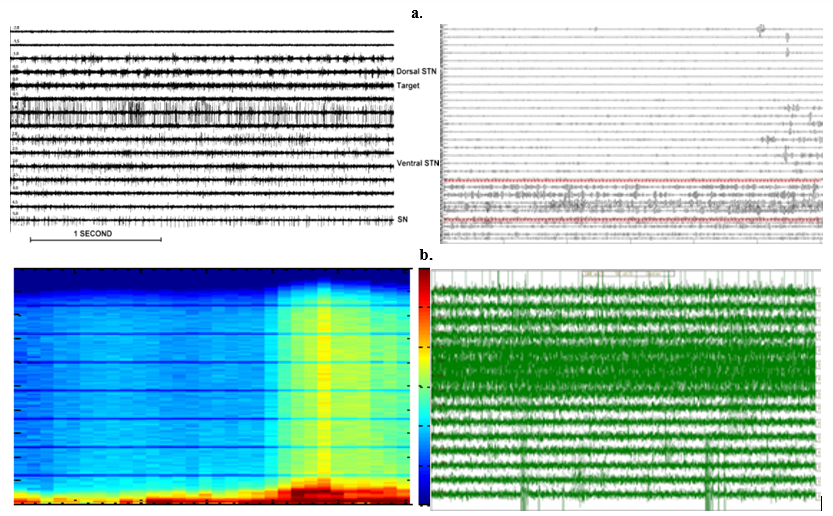
Figure 2
Inconsistencyversus depth plot for all ∀ -
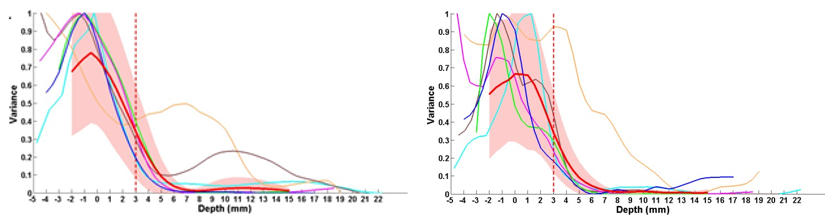
Figure 3
Depth versus variance scenario along with the variations amongst every successive datapoints for a typical PD-subject. Filtering with the band-pass filters at 13Hertz-30Hertz. Bluish Indigo navy-colored arcs depicting the variation. Red-faced pink curve depicting the variance of successive points in conjunction with the data. Dark pitch-black line is the 10 % limit.
Discussion
Earlier experiments indicated that the disproportionate extreme
Conclusion
We may infer that in conclusion, macro electrode signal acquisitions of STNs derived L F P gatherings might offer an unconventional methodology in the direction of m e r – s n a, for detecting the aimed target S T N perimeter-edges during DBS functional-neurosurgery.
