- Visibility 66 Views
- Downloads 5 Downloads
- DOI 10.18231/j.ijn.2021.017
-
CrossMark
- Citation
A brief review on efficacy and safety of brivaracetam in the management of partial onset epilepsy
- Author Details:
-
Gajanan Vithoba Panchal *
-
Vijay Nath Mishra
Introduction
According to WHO, epilepsy affects approximately 50 million people worldwide. 80% of these individuals are residing in developing regions. Around 10–12 million people with epilepsy reside in India. The prevalence ranges from 3.0-11.9/1000 population and incidence ranges from 0.2 - 0.6/1000/year in India.[1] Among main types of epilepsy, focal seizures (29.3%–63%) slightly predominant over generalized seizures (12.9%–48%), as reported in majority of studies.[2]
About 50% of patients with epilepsy (PWE) remain seizure free with single Antiepileptic Drug (AED) for at least 12 months and the remaining 50% patients need further treatment manipulation including substitution or combination therapy.[3] About 20–30% patients fall into drug-refractory epilepsy with use of ≥2 conventional AEDs in appropriate and adequate doses.[1] The goal of AED therapy is to ensure the best possible quality of life by maximizing seizure control and minimizing unacceptable medication related side effects.[4] Newer AEDs have evolved with the aim to tackle epilepsy with a different mechanism of action, while ensuring better safety profile and fewer drug-drug interactions. Moreover, cognitive profile and addressing the psychosocial and behavioral problems associated with epilepsy, have gained more attention, with the result that QoL has become the central focus of epilepsy care.[5]
This review aims to discuss pharmacological characteristics, details of efficacy, tolerability, and safety aspects of brivaracetam. A comprehensive literature search has been conducted for the published articles on brivaracetam in Google Scholar and PubMed database.
Brivaracetam
Brivaracetam, a propyl analog of levetiracetam, is a member of the racetam class of drugs, related to piracetam and Levetiracetam. Brivaracetam, is a new AED, and the first selective ligand that binds to synaptic vesicle protein 2A (SV2A). Brivaracetam was discovered during a target-based rational drug discovery program. 12,000 compounds were screened for SV2A binding affinity, only brivaracetam entered clinical trials. [6], [7]
Approved indications for brivaracetam
US: Both as monotherapy or adjunctive therapy for the treatment of partial-onset seizures in patients 4 years of age and older. Injection is indicated for the treatment of partial-onset seizures only in adult patients (16 years of age and older).[8]EU: As adjunctive therapy in the treatment of partial onset seizures with or without secondary generalisation in adults, adolescents, and children from 4 years of age with epilepsy. [9]India: As an adjunctive therapy in the treatment of partial onset seizures in patients 16 years of age or older.[10]
Dosage forms and strengths ([Table 1])
|
Strength |
Oral formulation: |
|
|
Film-coated tablets -25 mg, 50 mg, 75 mg, and 100 mg |
|
|
Solution -10mg/ml |
|
|
Intravenous formulation: |
|
|
Injection or infusion -50 mg/5 mL |
|
|
Initial Dosage: |
|
|
50 mg twice daily (100 mg per day) |
|
Recommended starting dosage of adjunctive Brivaracetam [8] |
Minimum and Maximum Maintenance Dosage: |
|
25 mg to 100 mg twice daily (50 to 200 mg per day) |
|
|
When initiating treatment: |
|
|
Gradual dose escalation is not required. Dosage should be adjusted based on clinical response and tolerability. |
Pharmacodynamics & Mode of action

Brivaracetam possess 15-30-fold higher and selective binding affinity to SV2A which is a transmembrane protein present on synaptic vesicles in endocrine cells and neurons which modulate neurotransmitter exocytosis.
Brivaracetam enters into recycling of synaptic vesicles. It produces a frequency-dependent decrease of synaptic transmission at 100-fold lower concentration and thus it more effectively slows the synaptic vesicle mobilization. [3]
The selective binding of brivaracetam exerts no direct effect on AMPA, GABA and glycine. Also, it has no effect on voltage-gated potassium channels or high- and low-voltage-gated calcium channels at therapeutically relevant concentrations ([Figure 1]).[7]
Pharmacokinetics ([Table 2]):$
Brivaracetam is absorbed rapidly and completely. It shows faster blood-brain barrier permeability within minutes. It shows low protein binding. Its major route of metabolism is hydrolysis followed by hydroxylation. Dose reduction is not required in renally impaired patients; however, there is no data available in patients with End Stage Renal Disease (ESRD). Dose reduction is required in patients with hepatic impairment.
|
Pharmacokinetics parameter |
Brivaracetam |
|
Absorption |
Absorbed rapidly and completely and can be administered with or without food. |
|
Bioavailability |
100% |
|
Permeability in brain |
Faster blood–brain barrier permeability within minutes (0.315 mL/min/g) |
|
Distribution |
0.5 l/kg. Brivaracetam is rapidly and evenly distributed in most tissues. |
|
Half life |
9 hours |
|
Protein binding |
Low plasma protein binding (17.5%) |
|
Time to steady state, after repeated administration |
2 days |
|
Metabolism |
• Major route: By hydrolysis of the acetamide group, leading to the production of a carboxylic acid metabolite, constituting 34% of urinary excretion. • Secondary route: Hydroxylation mediated by cytochrome P450 2C19 (CYP2C19) |
|
Elimination |
• Around 95% of metabolite elimination occurs via kidneys within 72 hours, with an unchanged fraction of 8–11% and 34 % of the dose excreted as the carboxylic acid metabolite. • Faecal excretion accounts for <1 % of the dose |
|
Hepatic impairment |
1/3rd dose reduction is required across all grades of hepatic impairment with maximal daily dose of 150 mg |
|
Renal impairment |
Dose reduction is not required. No data available in End Stage Renal Disease (ESRD) |
Drug-Drug interactions ([Table 3]):[6], [7]
Rifampicin reduces concentration of Brivaracetam and thus dosing may need increments. Co-administration of Brivaracetam with carbamazepine can result in increased concentration of carbamazepine metabolite. Supratherapeutic brivaracetam dose of 400 mg/day increase phenytoin exposure by approximately 20%. However, the recommended doses of brivaracetam are not predicted to have an effect on phenytoin exposure. Brivaracetam does not affect steady-state plasma concentrations of several commonly used AEDs such as carbamazepine, lacosamide, lamotrigine, phenobarbital, phenytoin, topiramate. Brivaracetam does not show clinically significant interaction with oral contraceptive containing ethinylestradiol and levonorgestrel.
|
Co-administered with Brivaracetam |
Drug-drug interaction |
|
Fluconazole and fluvoxamine (inhibitors of CYP2C19) |
Plasma concentration increases however, adverse clinical consequence is not likely. |
|
Rifampicin |
Rifampicin reduces the Brivaracetam area under the concentration curve and thus the dosing may need increments. |
|
Phenobarbital, Phenytoin and Carbamazepine |
• Slight reductions in plasma concentration of Brivaracetam. No dose adjustment is required. • Brivaracetam weakly inhibits phenytoin metabolism through CYP2C19 inhibition. Supratherapeutic brivaracetam dose of 400 mg/day can increase phenytoin exposure by approximately 20%. The recommended doses of brivaracetam are not predicted to influence phenytoin exposure. • Co-administration with carbamazepine showed 2.6-fold increase in carbamazepine epoxide concentrations (minimal clinical relevance). |
|
Ethinylestradiol and Levonorgestrel |
A 20–30% reduction occurred with Brivaracetam 400 mg/day without impact on suppression of ovulation. No interactions were observed with a dose of 100 mg/day. The interaction is not expected to be of clinical significance |
|
Acyclovir, Bumetanide, Ciprofloxacin, Zidovudine, Pravastatin, Rosuvastatin, Sitagliptin, Famotidine, Furosemide, Penicillin G, Methotrexate |
Concentrations of these transmembrane protein organic anion transporter OAT3 may be reduced by 200 mg daily of Brivaracetam. |
|
Carbamazepine, Lacosamide, Lamotrigine, 10-hydroxyoxcarbazepine, Phenobarbital, Pregabalin, Phenytoin, Topiramate, Valproate, Zonisamide |
Brivaracetam does not affect steady-state plasma concentrations of these AEDs. |
Efficacy profile
Brivaracetam shows early time to onset of sustained (≥50%) responder status
In pooled analysis (n=1160) of three phase-3 studies of brivaracetam (NCT00464269, NCT00490035, and NCT01261325), time to onset of sustained ≥50% responder status (SRS) was assessed. Early ≥50% responder rate (on day 1) was found across all brivaracetam treatment groups i.e. 15.5%, 18.1%, 19.4%, for 50, 100, 200 mg/day, respectively compared to placebo (6.7%) (p<0.001). The percentages of patients achieving sustained ≥50% responder rate (SRR) increased over time for all treatment groups, which were consistently greater for brivaracetam treated than placebo-treated patients (p ≤ 0.003 vs. placebo for all comparisons). The percentage of patients achieving SRR increased over time, indicating that efficacy was also sustained for patients who responded later in brivaracetam treatment. Overall ≥50% responder rates were 34.2%, 39.5%, and 37.8% for brivaracetam 50, 100, and 200 mg/day, respectively, versus 20.3% for placebo. This study demonstrates brivaracetam has a fast onset of action possibly due to rapid brain penetration.[13]
No dose titration required while initiating brivaracetam
Brivaracetam was started at target dose without titration in the three Phase III studies (N01253, N01252, N01358). These three pivotal studies evaluated fixed dosing of adjunctive brivaracetam (5–200 mg/day) with no up-titration over a 12-week treatment period. The patients enrolled were adults with uncontrolled focal seizures treated with one or two AEDs. Initiation of brivaracetam at the target dose without titration is possible due to rapid brain penetration and favorable tolerability profile of brivaracetam. Thus, brivaracetam is easy to use as it can be initiated at target dose without dose titration.[7]
Long-term, consistent seizure control with adjunctive brivaracetam
In a 11-year, open-label, follow-up trial by O'Brien JT et al. 2020 in patients with partial onset epilepsy, brivaracetam resulted in reduction of median focal seizure frequency/28 days from 9.2 (range = 0-17.304) to 4.2 (range = 0-11.726) with overall 57.3% reduction which Increased by exposure through 3 years and remained stable through 9 years. 50% responder rate was 55.6% (360/648) which also increased by exposure through 3 years and remained consistent through 9 years. Seizure freedom rates were 30.3% (170 patients) at 6 months and 20.3% (114 patients) at 12 months. This shows that brivaracetam was effective and well tolerated through the 9-year period and efficacy outcomes improved with increasing duration of brivaracetam exposure.[14]
In a long-term follow-up study over 5-years involving 2,051 (93.86%) of 2,186 patients who received brivaracetam and completed their phase IIb/III studies (May 2005 and May 2014), percentage of patients with a ≥50% reduction in Partial Onset Seizure (POS) frequency increased from baseline over 5 years, 43.5% (at 1 – 3 months) and 71.0% (at 58–60 months). Good retention rate was recorded for brivaracetam i.e. 91.0%, 79.8%, 68.1%, 54.4% at 6, 12, 24 and 60 months, respectively. The study concluded that adjunctive brivaracetam treatment in adults with partial onset seizures was effective and well tolerated over long term use (≥5 years) with high retention and improvements in health related QoL.[15]
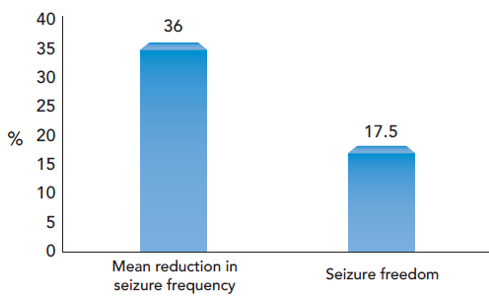
Villanueva V. et al. 2018 was a real real-life setting 1-year, retrospective study (BRIVA-LIFE) on brivaracetam involving 575 patients, conducted across 18 centers. Primary reasons for initiating brivaracetam were a) Inadequate seizure control (n=466, 81.1%) b) Inadequate seizure control combined with poor tolerability (n = 64, 11.1%) c) Poor tolerability (n=42, 7.3%). In this patient population, brivaracetam reduced mean seizure frequency by 36.0% at 12 months. Seizure‐freedom was observed in 17.5% of patients as shown in figure 2. ≥50% reduction in seizure frequency was recorded in 42.4%, 40.0%, 39.7% at 3, 6, and 12 months, respectively. High retention rate was also found i.e. 90.8%, 80.2%, 70.4% at 3, 6, and 12 months, respectively. In this study involving patients predominantly with drug‐resistant epilepsy, brivaracetam was found to be effective and well‐tolerated over 1 year.[16]
Brivaracetam: The most effective and acceptable AED in patients with focal drug-resistant epilepsy
Hu TY et al. 2020 was a network meta-analysis of 62 randomized controlled trials (n=12,739) to compare and rank the efficacy and acceptability of new AEDs for patients with focal drug-resistant epilepsy. This meta-analysis considered published double-blind RCTs which compared new AED as adjunctive therapy for focal-onset drug-resistant epilepsy against placebo or other AEDs including brivaracetam, carisbamate, eslicarbazepine, ezogabine/retigabine, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, perampanel, pregabalin, remacemide, rufinamide, topiramate, vigabatrin and zonisamide. Among the studied newer AEDs, brivaracetam was found to have highest seizure free rate and least discontinuation rate. Based on these outcome parameters, brivaracetam was identified as the most effective and acceptable treatment for focal drug-resistant epilepsy compared to newer AEDs.[17]
Efficacy, safety, and tolerability of adjunctive brivaracetam for focal seizures in elderly patients
This pooled analysis by Brodie MJ et al. 2016 aimed to assess the efficacy, safety, and tolerability of brivaracetam (50–200 mg/day) in 30 elderly patients aged ≥65 years. In this pooled analysis, median percent reduction from baseline in focal seizure frequency/28days by brivaracetam was 25.5%, 49.6%, and 74.9% for brivaracetam 50, 100, and 200 mg/day, respectively vs 14.0% by placebo. ≥50% responder rate for brivaracetam was 25.0%, 50.0%, and 66.7% for 50, 100, and 200 mg/day, respectively vs. 14.3% for placebo. The most commonly reported treatment emergent adverse effects (TEAEs) were headache, paresthesia and somnolence. The study suggested that brivaracetam may be a suitable adjunctive treatment in older patients with uncontrolled focal seizures.[18]
Adjunctive brivaracetam showed higher responder rates and better adverse effect profile among newer AEDs in patients with refractory partial onset epilepsy: Meta-analysis
The meta-analysis of 15 RCTs by Gao L. et al. 2013 aimed to evaluate the clinical efficacy and safety of AEDs namely, eslicarbazepine, retigabine/ezogabine, carisbamate, lacosamide, brivaracetam and perampanel as adjunctive therapy for adults with refractory partial onset seizures. The indirect comparisons based on pooled Odds Ratio (ORs) suggested that brivaracetam might be more effective than all other newer AEDs in terms of highest responder rates, lowest incidence of withdrawal rates and lowest incidence of adverse effects.[19]
Safety and tolerability profile
Effects of brivaracetam on cardiac repolarization: A thorough QT study
The study by Rosillon D et al. 2008 evaluated the effect of brivaracetam on cardiac repolarization in healthy subjects. Subjects in this study received double-blind treatment with brivaracetam 75 mg bid (n=39), brivaracetam 400 mg bid (n=40) or placebo bid (n=53), or open-label single-dose moxifloxacin 400 mg (n=52). Continuous ECG recording was conducted at baseline and after giving the last dose. In this study, brivaracetam showed no effect on cardiac repolarization at both therapeutic (150 mg/day) and supratherapeutic (800 mg/day) doses. This study suggests that brivaracetam therapy may not require intensive cardiac monitoring.[20]
Brivaracetam shows lower incidence of behavioral/psychiatric adverse effects, hypersensitivity reactions and hepatotoxicity
Psychiatric and behavioral AEs are common in patients taking AEDs. Psychiatric comorbidities can be found in 25-50% of patients with epilepsy, with higher prevalence among patients with poorly controlled seizures. Brivaracetam has been found to have lower incidence of behavioral/psychiatric adverse effects.[21]
A phase III study by Biton V. et al. 2013 of brivaracetam (5, 20, or 50 mg/day) as adjunctive treatment for uncontrolled partial epilepsy in adults reported following psychiatric disorders ≥1% of patients: insomnia (brivaracetam 4.0% vs. placebo 2.0%), depression (brivaracetam 3.7% vs. placebo 1.0%), irritability (brivaracetam 3.7% vs. placebo 2.0%), anxiety (brivaracetam 1.7% vs. placebo 1.0%), memory impairment (brivaracetam 1.7% vs. placebo 1.0), agitation (brivaracetam 1.0% vs. placebo 0%), and depressed mood (brivaracetam 1.0% vs. placebo 0%). This study showed that adjunct brivaracetam therapy at dose up to 50 mg was well tolerated with relatively lower incidence of psychiatric adverse effects.[22]
In a Real-life study by Steinhoff BJ et al. 2017, 101 patients were treated with add-on brivaracetam at doses between 50 mg and 400 mg daily. Treatment Emergent Adverse Effects (TEAEs) occurred in 37% of the patients. Leading adverse events were dizziness (16%) and somnolence (11%) while psychiatric adverse event occurred only in a single case.[23]
In order to provide comprehensive safety data for adjunctive brivaracetam in patients with focal onset epilepsy, Brandt C. et al. 2020 analyzed the data pooled from two phase-II and four phase-III clinical studies on brivaracetam. 1271/1957 patients received brivaracetam and 686 received placebo. This analysis reported considerably low incidence of behavioral disorder related TEAEs (4.0% with brivaracetam vs. 2.5% with placebo). Irritability was observed in 2.7% of brivaracetam-treated patients vs. 1.5% of patients receiving placebo. ≤1% of patients receiving brivaracetam reported anger, aggression, and agitation. Treatment-emergent adverse events associated with psychosis were psychotic disorder and were found in three patients on brivaracetam vs. two patients on placebo.
In the above study by Brandt C. et al. 2020, no patients reported TEAEs of bronchospasm or angioedema, or any TEAEs associated with anaphylaxis. Rash and pruritus occurred in 1.2% (15/1271) and 1.4% (18/1271) patients receiving brivaracetam, respectively compared with 1.3% (9/686) and 1.0% (7/686) patients receiving placebo. TEAEs associated with hepatotoxicity for brivaracetam was 1.9% (24/1271) and placebo-treated was 1.6% (11/686).
Brivaracetam improved anxiety and QoL
Lifetime prevalence of depression associated with epilepsy has been reported to be as high as 55%. Among patients with recurrent seizures, 20–30% patients are found to be having depression.[24] Inter-ictal anxiety disorder is estimated to be prevalent in up to 25% of patients with epilepsy.[25] More than 50% of epileptic patients have been found to have a moderate to poor QoL.[26] Thus, there is a considerable burden of anxiety and depression in patients with epilepsy leaving them with poor quality of life.
In long-term follow-up trial by O’Brien TJ et al. 2020, treatment with add-on brivaracetam in patients with epilepsy (n=667) reported improvement in anxiety based on Hospital Anxiety and Depression Scale scores (HADS). Over 2-year evaluation showed mean changes from baseline in HADS as -0.7 (standard deviation [SD]: 4.3) for anxiety and -0.2 (SD: 4.4) for depression indicating improvement in anxiety while depression score showed minor decrease from baseline over time. Quality of Life of patients was also found to be improved based on Patient‐Weighted Quality of Life in Epilepsy Inventory (QOLIE‐31‐P) score.[27]
Adverse Effects
Both oral and intravenous forms of brivaracetam are found to be generally well tolerated as an adjunctive therapy over long term use. The most frequently reported TEAEs associated with brivaracetam were somnolence, dizziness, fatigue, and headache. Brivaracetam, shows lesser incidence of psychiatric/behavioral adverse events (irritability, insomnia, anxiety, depression) Also, brivaracetam has lower incidence of hypersensitivity reactions like skin rash (1.2%) and pruritus (1.4%).[6], [28]
Conclusion
In patients with epilepsy, the goal of antiepileptic drug therapy is to maximally reduce seizure, minimize side effects of medication and ensure best possible quality of life. Newer Antiepileptic Drugs have been developed with the objective to tackle epilepsy with different mechanism of action, better safety profile and fewer drug-drug interactions. Brivaracetam, a propyl analog of levetiracetam shows 15-30-fold high and selective affinity for synaptic vesicle 2A. It has high lipid solubility and faster brain penetration. When initiating treatment with brivaracetam, gradual dose escalation is not required. Thus, right therapeutic dose can be administered on first day itself. Brivaracetam demonstrates rapid onset of action, early and sustained seizure control with good retention rate. Brivaracetam has better safety profile with most frequently reported side effects such as somnolence, dizziness, fatigue, and headache. Brivaracetam shows minimal drug-drug interactions and lesser incidence of behavioral and psychiatric adverse effects. Brivaracetam exhibits improvement in anxiety, cognitive profile, executive functions and quality of life of patients with epilepsy.
Conflict of Interest
The authors declare that there is no conflict of interests
Source of Funding
None.
References
- D Garg. Specific considerations for epilepsy in India. Curr Med 2020. [Google Scholar]
- G Coppola, G Iapadre, FF Operto, A Verrotti. New developments in the management of partial-onset epilepsy: role of brivaracetam. Drug Des Devel Ther 2017. [Google Scholar] [Crossref]
- LJ Stephen, MJ Brodie. Antiepileptic drug monotherapy versus polytherapy: pursuing seizure freedom and tolerability in adults. Curr Opin Neurol 2012. [Google Scholar]
- P Perucca, IE Scheffer, M Kiley. The management of epilepsy in children and adults. Med J Aust 2018. [Google Scholar] [Crossref]
- A Radhakrishnan. Bridging the treatment gap in epilepsy-is there an emerging trend in the use of newer antiepileptic drugs?. Neurol India 2016. [Google Scholar]
- AM Feyissa. Brivaracetam in the treatment of epilepsy: a review of clinical trial data. Neuropsychiatr Dis Treat 2019. [Google Scholar] [Crossref]
- P Klein, A Diaz, T Gasalla, J Whitesides. A review of the pharmacology and clinical efficacy of brivaracetam. Clin Pharmacol 2018. [Google Scholar] [Crossref]
- . Briviact (Brivaracetam) Prescribing Information. 2018. [Google Scholar]
- . Briviact (Brivaracetam) SmPC . . [Google Scholar]
- . . . [Google Scholar]
- J M Nicolas, J Hannestad, D Holden. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 2016. [Google Scholar]
- SM Hoy. Brivaracetam: A Review in Partial-Onset (Focal) Seizures in Patients with Epilepsy. CNS Drugs 2016. [Google Scholar]
- P Klein, ME Johnson, J Schiemann, J Whitesides. Time to onset of sustained ≥50% responder status in patients with focal (partial-onset) seizures in three phase III studies of adjunctive brivaracetam treatment. Epilepsia 2017. [Google Scholar] [Crossref]
- TJ O’Brien, S Borghs, Q (Jane) He, AL Schulz, S Yates, V Biton. Long‐term safety, efficacy, and quality of life outcomes with adjunctive brivaracetam treatment at individualized doses in patients with epilepsy: An up to 11‐year, open‐label, follow‐up trial. Epilepsia 2020. [Google Scholar] [Crossref]
- M Toledo, J Whitesides, J Schiemann, ME Johnson, K Eckhardt, B McDonough. Safety, tolerability, and seizure control during long‐term treatment with adjunctive brivaracetam for partial‐onset seizures. Epilepsia 2016. [Google Scholar] [Crossref]
- V Villanueva, FJ López-González, JA Mauri, JR Uranga, M Olivé-Gadea, J Montoya, . BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta Neurologica Scandinavica 2019. [Google Scholar] [Crossref]
- T Y Hu, H Q Wang, W P Zhang, R F Tian, G S Lei, Y C Deng. Network meta-analysis of antiepileptic drugs in focal drug-resistant epilepsy. Epilepsy Res 2020. [Google Scholar] [Crossref]
- MJ Brodie, J Whitesides, J Schiemann, J D’Souza, ME Johnson. Tolerability, safety, and efficacy of adjunctive brivaracetam for focal seizures in older patients: A pooled analysis from three phase III studies. Epilepsy Res 2016. [Google Scholar] [Crossref]
- L Gao, L Xia, F L Zhao, S C Li. Clinical efficacy and safety of the newer antiepileptic drugs as adjunctive treatment in adults with refractory partial-onset epilepsy: a meta-analysis of randomized placebo-controlled trials. Epilepsy Res 2013. [Google Scholar]
- D Rosillon, B Astruc, R Hulhoven, MA Meeus, MM Troenaru, S Watanabe. Effect of brivaracetam on cardiac repolarisation – a thorough QT study. Curr Med Res Opin 2008. [Google Scholar] [Crossref]
- WC Lafrance, AM Kanner, B Hermann. Psychiatric comorbidities in epilepsy. Int Rev Neurobiol 2008. [Google Scholar]
- V Biton, SF Berkovic, B Abou‐Khalil, MR Sperling, ME Johnson, S Lu. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: A phase III randomized, double‐blind, placebo‐controlled trial. Epilepsia 2014. [Google Scholar] [Crossref]
- BJ Steinhoff, M Bacher, I Bucurenciu, B Hillenbrand, T Intravooth, R Kornmeier. Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy—A monocenter survey. Seizure 2017. [Google Scholar] [Crossref]
- MJ Jackson. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry 2005. [Google Scholar] [Crossref]
- O Y Kwona, S P Park. Depression and Anxiety in People with Epilepsy. J Clin Neurol 2014. [Google Scholar]
- A Gholami, S Salarilak, P Lotfabadi, F Kiani, A Rajabi, K Mansori. Quality of life in epileptic patients compared with healthy people. Med J Islam Repub Iran 2016. [Google Scholar]
- TJ O’Brien, S Borghs, Q (Jane) He, A‐L Schulz, S Yates, V Biton. Long‐term safety, efficacy, and quality of life outcomes with adjunctive brivaracetam treatment at individualized doses in patients with epilepsy: An up to 11‐year, open‐label, follow‐up trial. Epilepsia 2020. [Google Scholar] [Crossref]
- C Brandt, P Klein, V Badalamenti, T Gasalla, J Whitesides. Safety and tolerability of adjunctive brivaracetam in epilepsy: In-depth pooled analysis. Epilepsy Behav 2020. [Google Scholar] [Crossref]
- Introduction
- Brivaracetam
- Approved indications for brivaracetam
- Dosage forms and strengths ([Table 1])
- Pharmacodynamics & Mode of action
- Pharmacokinetics ([Table 2]):$
- Drug-Drug interactions ([Table 3]):[6], [7]
- Efficacy profile
- Brivaracetam shows early time to onset of sustained (≥50%) responder status
- No dose titration required while initiating brivaracetam
- Long-term, consistent seizure control with adjunctive brivaracetam
- Brivaracetam: The most effective and acceptable AED in patients with focal drug-resistant epilepsy
- Efficacy, safety, and tolerability of adjunctive brivaracetam for focal seizures in elderly patients
- Adjunctive brivaracetam showed higher responder rates and better adverse effect profile among newer AEDs in patients with refractory partial onset epilepsy: Meta-analysis
- Safety and tolerability profile
- Adverse Effects
- Conclusion
- Conflict of Interest
- Source of Funding
