- Visibility 661 Views
- Downloads 63 Downloads
- Permissions
- DOI 10.18231/j.ijn.2020.041
-
CrossMark
- Citation
MER based analysis of local field potentials with deep brain stimulation subthalamic nucleus in Parkinson/'s disease using coherence and entropy techniques
- Author Details:
-
Venkateshwarla Rama Raju *
-
Srinivas Konda
-
Kavitha Rani Balmuri
-
Anvesh Balabhadra
-
BSV Raju
Abstract
Deep brain stimulation (DBS) is a device-based well-developed and well-established innovative frontier surgical-therapeutic method which reduces the symptoms of Parkinson`s disease (PD) and restores and increases the motor-functioning. DBS gives a unique-opportunity to study the electrical-oscillatory(harmonic-ripples) neural-activity of various sub-cortical-structures in PD-subjects. However, the electrically-stimulating local field potentials (LFPs) are fundamentally concerned during subthalamic-nuclei (STN) recording. The fluctuations, measured to signify collective neuronal-discharge from neurons surrounding the electrode. The acquisition of extracellular activity of irregular-patterns of STN-activity typically acquired from a population-of-neurons detected as” local field potentials” has discard luminosity on the pathophysiology and seizes the latent to pilot to elegance in modern DBS management. The recordings are often gathered with either intraoperative microelectrode for neuronal-activity and/or DBS-leads for chronic-macro-stimulation and reflect oscillatory-activity within nuclei of the basal-ganglia (BG) and thalamic-targets for diagnosing PD. LFP-recordings have numerous clinical implications and presently used to optimize DBS outcomes in closed-loop adaptive-devices/systems. However, the origins of the LFPs are implied softly and implicitly. Thus, the goal of this present study is to analyze LFP recordings within the milieu of clinical-applications for clinical-significancy and this goal is attained with frequency analysis ranging the band from 1Hz-250Hz and coherence band between 0 and 1 level. The results of the study suggest that the spatial-reach of the LFP can extend several millimeters. This study presents a comprehensive investigation into the existing research which gives insights into the origin of LFP-signals and identify the variables that need to be considered when analyzing LFP-signals in clinical settings principally DBS-applications. Dependable-correlations between motor-features and the mechanisms of the LFP power-spectra (the power-spectral-density, PSD) imply that LFPs may serve as biomarkers (biosignals) for movement-disorders (MDs) as a clinical-relevance. In particular, the cardinal motor-feature has been shown to correlate with β-fluctuations and tremor cohered between 8Hz-28Hz. Thus, the local field-potential connotations are for enhanced electrode-targeting and for the development of a multi-channel/real-time and thus online, personalized adaptive/closed-loop-systems. Variables like geometry-of-the-electrode/recording-configuration can have a significant-effect on LFP-amplitude pulse-width, stimulus-intensity and spatial-reach, whilst the effects of other variables, like electrode-impedance, are often trifling. Entropy was measured in all 12 patients (right-hemisphere with DBS “on” = 1.4±0.1; DBS off: 1.4±1.9; and left-hemisphere on: 1.5±0.1 and off: 2.3±1.2) for tremor-complexity while root mean square measured for amplitude. For the data consistency, coherence was applied to see the variation (inconsistency) and irrationality (if any) which was a normalized measure of linear association in frequency domain where in the bounded-measure was between 0 and 1. If it is more than 0.75 but less than or equal to 1 (i.e., > 0.75 ≤ 1 = coherence) there is linear association else no coherence. In our computation, we obtained coherence > 0.75.
Introduction
Parkinson`s disease (PD) is a chronic intricate and progressive neurodegenerative disease which is differentiated by the convolution of a broad spectrum of components called “cardinal-motor-manifestations (or symptoms)”. Based on clinical-prognostic estimation, the cardinal-motor-manifestations were categorized into four classes of features, namely, tremor, Bradykinesia, postural instability and rigidity using the score of the Unified Parkinson`s Disease Rating Scale (UPDRS)[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19] followed by the amendments’ incorporated in UPDRS scales (such as, UPDRS-III, III+, etc) and also as per United Kingdom Parkinson disease society brain bank (UKPDS-BB) and specific phenotype quantifications.[20] The main pathology of Parkinson’s disease is present in the nigrostriatal system which is distinguished by the corrosion and/or erosion (or oxidization) of the dopaminergic-neurons in the substantia-nigra ([Figure 1]).
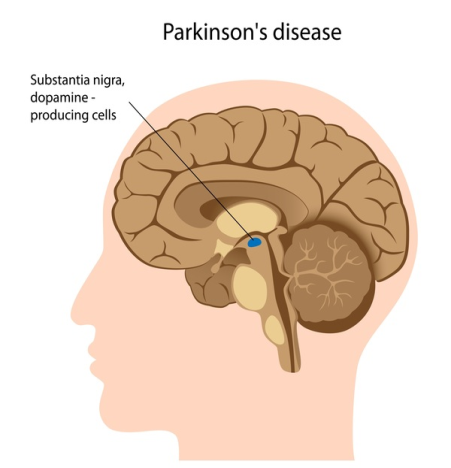
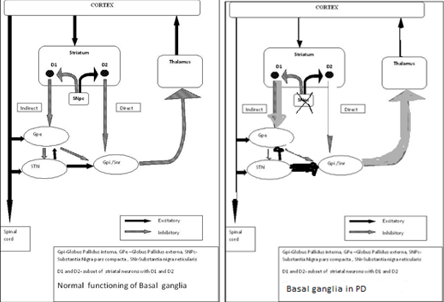
Substantia-nigra pars-compacta (SNpc) is an important element of the basal-ganglia (BG) circuitry which modulates the cortex and helps in fine tuning motor activities. Two dopaminergic-pathways that are involved form the striatum to the thalamus and the cortex- direct pathway which leads to stimulation of the cortex and indirect pathway which inhibits the cortex. The dopaminergic-supply from the SNpc acts by D1 receptors which activate the direct-pathway and the D2 receptors which inhibit the indirect-pathway. Absence of these neurons leads to an increased firing from the subthalamic-nuclei (STN) and globus-pallidal interna (GPi) neurons which lead to augmented inhibition of the thalamic-neurons and cortex and overall reduced movement [1]. [Figure 2] a,b, illustrate the functioning of normal basal-ganglia (BG) circuitry and dysfunctioning of normal BG-circuitry and hence the abnormality in idiopathic Parkinson’s disease.
(a) Basal Ganglia Circuitry in a normal subject (b) Parkinson`s disease in an abnormal subject
The beginning of the medical-drug-management by early levodopa was followed by the armamentarium of diverse-drugs but the medical-administration is hampered by the manifestation of a variety of side-effects, such as, dyskinesias and on-off phenomenon. Though dopaminergic-drive in normal PD-subject is stable, the oral-prescriptions cannot fully impersonate the normal-control-state through concentric-medical drug varying from trough to peak levels based on the time of consumption.
Scientists have discovered that, in various cases, the occurrence or incidence of Parkinson disease emerges to be unpredictable and also impulsive. Numerous risk-factors have been-documented, amid most age of life-form, collectively with ‘genetics’ (the “genetically-hereditary”) and surrounding-atmosphere ‘environment’. While genetic-risk-factors are being defined, the genetics of Parkinson’s disease are intelligibly not unambiguous or comprehensible and the symptoms of these genetics are said to be “genotype.” Some researchers think that the combination of genetics and environment might actually speed up a normal aging trajectory. Neuroscientists achieve that there is injury at a position otherwise be deficient of functioning of these dopamine-cells at a peak-position in human-life. However, scientists are not certain at what-place or where-it be or anticipated and for how long it goes on persist. Therefore, the only remedy/therapeutic-medication is to predict the symptoms in the beginning itself which facilitates us to comprehend growth of this malady as it be other than these dopamine-cells in the brain and it affects other cells as well.
Further, scientists have established that the “malady” arises from insufficient-quantities of the neurotransmitter-dopamine in an area of the brain that controls-movement, the “basal-ganglia” which is a parallelly-connected-distributed-circuit. [21], [22]
George Cotzias (Lasker Clinical Medical Research Award, 1969) had reported dramatic improvements in PD patients who received a carefully tuned regimen of oral levodopa (L-dopa, the metabolic precursor of dopamine). However, the drug induced severe involuntary-movements in some individuals. Only small windows of the day remain in which patients experience neither PD symptoms nor these disturbing effects.
Work done by Benabid[21] and DeLong [22] has provided insights into well-established observation that cognitive and emotional problems accompany many motor-disorders originating from failure of BG circuit. Furthermore, their findings provided a new framework for exploring how BG elements malfunction in various illnesses, including PD. Although dopamine loss-clearly causes the disease`s motor perturbations, the allied changes in BG activities were ambiguous and thus motor disturbances feature predominantly. De Long`s model which included detailed road maps of stimulatory and inhibitory signals through the basal-ganglia offered concepts, ideas, notions and perceptions. With the introduction of STN-DBS. [21], [23] the experimental investigations are conducted in two ways. Through medication (levodopa, etc) drug trial, and through interventional study (STN, GPe) lesion identification signal and imaging ultrasound, such that the following objectives can be met: Patterns of symptoms of individual patients are too complex for standardized treatment, so need to predict and prevent side effects, find best therapeutic response, and find out neuroprotective therapies. This study conducted using second method. While some progress was made by medication side particularly on cognitive dementia, not much work was done in the area of non motor symptoms with interventional study.
Deep brain stimulator (DBS) is an innovative frontier surgical therapeutic technological method for maximally uprooting symptoms of Parkinson’s disease (PD) and other movement disorders (MDs) as well. It gives a unique opportunity to study the electrical-oscillatory (harmonic-ripples) neural-activity of various sub-cortical (deep) brain structures in PD-MDs subjects. [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13] However, the electrically stimulating local field potentials are a great concern during subthalamic-nuclei (STN) recording. LFP’s are fluctuations, measured to signify collective neuronal-discharge from neurons neighboring the electrode. The acquisition of extracellular activity of irregular patterns of STN activity typically acquired from a population of neurons detected and defined as local field potentials (LFPs) has discard luminosity on the pathophysiology and seizes the latent to pilot to elegance in modern management. [8] DBS is a stereotactic functional neurosurgical procedure principally entrenched by a neurologist and surgery by a qualified and suitable surgeon for functionally implanting electrodes into a predetermined target region based on the signs and symptoms being treated by surgery. The target coordinates are derived, confirmed based on pre operative magnetic resonance imaging (MRI), and customized through electrophysiological microelectrode-recording (MER) technique while stimulating electrode deeply into the important part of the brain, intraoperatively [8]. The technique allocates the detection of neurons which are characteristic-features of the concern target during firing-rate, voltage-amplitude and signature-patterns. For instance, through MER, the subthalamic-nuclei (STN) is usually detected with setting-noise, increased firing-rate and frequency of bursting-neural-cells. Consequent upon, the DBS electrode is implanted based on the patterns (or signatures) found by MER. Both the MER and DBS electrode leads are capable of recording LFPs. The acquired signal is referred to as a ‘biomarker’ which can vary in accordance with the nature, for instance, biomedical, bioelectrical, biochemical, neurological, physiological, and biological.
Local field potentials are widely employed feed-back responsive signals/waveforms in adaptive closed loop DBS systems [24], [25], [26] that are also referred to as ‘intracranial electroencephalograph-waveforms’ computed-generated from the extracellular-space by transmitting electrical-potentials (the action potentials) in the course of axons. These field-potentials are often replicate neuronal procedures happening within the local-region around the electrode in the neuronal-extracellular-space. Priori et al[27] established the suitability of LFPs as the feedback signal in the adaptive DBS systems for Parkinson disease subjects. A key advantage is that LFPs can be directly acquired from the stimulating electrodes. The other advantage is the long term constancy attained at the electrode-tissue-interface. [28] Typically LFPs have amplitudes of up to 200 micro-volts (µV) with energies less-than 500Hz.[26] In contrast to electrical-potentials (the action-potentials), local-field-potentials have a reasonable spatio-temporal resolutions, classically around 1millimeter.[29] So far, several, indeed many neurological movement disorders have been investigated with adaptive DBS devices tamed by local field-potentials [30]. While Parkinson disease and epileptic-seizures have been meticulously focused on other symptoms, such as, tremor, dementia, depression; and seizure parameters like hemisphere duration, etc., the selection of an appropriate biomarker depends on several factors: Typically, chosen with respect to the disease-type and the degree of relevance to the features of the disease, apart from being coupled with symptom features, it is critical and imperative for a LFP signal to be acquired with a signal to noise-ratio (S/NR), and most importantly be able and unaffected by external ripples, like speaking, thinking, heaving, moving and waving.[31]
Microelectrode recording (MER) or microelectrode signals recording of local field potentials with subthalamic-nuclei deep brain stimulation is most useful for interpreting Parkinson diseases (PD) signal analysis acquiescent to elucidation are fetching ever more germane or pertinent. These signals are supposed to emulate STN neurons and action potential movement and, these potential frequency modulations are coupled to spiking-events.
Also, the existing microelectrode-recording (MER) data vis-à-vis the neuro-electro-physiological (NEP) irregularities (of uncharacteristic) in the subthalamic-nuclei which probably lead acuteness of the PD features (i.e., the non-genetic phenotype PD symptoms) are very scanty. In this study, we[2], [3], [4], [5] state that activity in associated-bands of local field potentials (LFPs) gathered with multi-channel subcutaneous microelectrode recording system and DBS leads from sub-territories of STN give characteristic neurophysiological information about the PD symptoms-indications.
Subthalamic-nucleus deep brain stimulation (STN-DBS) is an effective treatment for advanced Parkinson’s disease (PD). In this, there are two electrophysiological techniques sustain detection of the optimal target. One is to record neuronal activity with microelectrodes (MER). The other is to record the local field potential (LFP) from the DBS electrode used for chronic stimulation. The relative predictive value of the two techniques is to be established. We explore whether there is any advantage in combining intraoperative LFP techniques with MER. High frequency deep brain stimulation (DBS) in the STN area has proven to be an effective treatment for patients with advanced PD. Surgical targeting of the area is by and large facilitated by MER of single units and milieu neuronal activity. An auxiliary technique has been promoted as a candidate intraoperative aid for targeting the optimal stimulation site along the planned DBS electrode trajectory and selecting the best contacts for chronic stimulation.
Twelve PD subjects with two groups were recruited in this study and found distinct patterns between two groups (n=8 in concordant group A, n=4 in discordant group B, during high frequency, fluctuations (HFFs) and their nonlinear interactions with beta (β)-band in the advanced and mediocre regions of the STN. Power of the slow high frequency fluctuations (sh-fOs, 200Hz-260Hz) are predominant and the blending of its voltage with β-band phase were significantly stronger in group A. the mediocre region of STN exhibited fast HFFs (fHFFs, 260Hz-450Hz), which have a significantly upper center frequency in the B group. The cross-frequency blend between fHFFs and β-band in the mediocre region of STN was significantly stronger in the A group. The results show that the spatio-spectral dynamics of STN-LFPs can be used as an objective method to differentiate these two aiming the associated-territories of STN for the personalization of DBS techniques. In this study, it is seen how the β-fluctuations in the STN-DBS enhance our understanding clinically in prognostic diagnosis of recordings of local field potentials. This study was approved by the institute ethical committee following Helsinki principles.
Aims and Objectives
To implant the pulse generators and acquire the local field potentials during subthalamic-nuclei (STN) recording and to analyze these potentials.LFP signal-recording.
Hypothesis/Rationale
Parkinson’s disease is a major movement disorder and the prime root cause is damage to the central-nervous –system(CNS). In spite of every study on this malady, the formation-mechanism of its manifestations remained mysterious. But it is quiet obscure why damage to substantia nigra only which is, a tiny element of the brain (few millimeters), causes a wide-range of motor-symptoms? Still, the basic reasons of brain damages or injuries prolong to be wholly expounded and also understanding the brain function is very impracticable and may be unachievable. Some cutting edge frontline engineering and technological tools and utilities are un-soothing to comprehend the behavioral actions and activities and performance of complex-systems [32]. In this connection, mathematical frameworks and statistical signal modeling and then simulation modeling’s through computer (i.e., computer simulated prototypes through computational simulation techniques) is one of the most imperative-tools. Computational simulation models for the progression of this malady have begun in 1999 and today it is expanded profoundly. These engineering developmental tools are very helpful not only in improved perceptive and thoughtful of the PD, but also presenting innovative therapeutic-methods, and it’s envisage prediction and impediment, and in its early diagnosis.
The goal is to find out if an adaptive (closed-loop) DBS system — responding to patient-specific, clinically relevant brain or movement signal feedback — is more effective than the currently available, open loop analog DBS therapy in Parkinson’s disease (PD) as précised by the motor-score on the Unified Parkinson’s Disease Rating Scale (UPDRS III), and also, as per United Kingdom Parkinson disease society brain bank (UKPDS-BB) criteria and specific phenotypic quantifications.
Rationale
At present DBS is constrained to non-adaptive open loop stimulus method, without automatic adjustments or settings to the subject’s activity status, fluctuations plus kinds of motoric-features, drug-medication (i.e., dosages) or neural markers of the disease. Adjustments of stimulation parameters are not conducted during real-time in real-time based on the ongoing neurophysiological variations in the brain. Hence, adverse effects on patient may be induced due to overstimulation of the brain. Whilst subject at home, every adjustment to DBS settings occur or during visits. Such constraints sometimes may lead to further health hazards and cross side-effects, like dyskinesia, cognitive dementia(CD) and cognitive impairment (CI), feelings, the doldrums, hallucinations, and both upper body symptoms (such as, dysarthria, deglutition, and respiratory-function) and lower body symptoms (like gait-disorders, freezing-of-gait (FoG) and including symptoms associated-groups like tremor-dominant (TD) and postural-instability gait-difficulty (PIGD)) and axial symptoms. These features have a key impact on disease-progression, and thus, the subjects’ quality-of-life (QoL) and the encumbrance of caregiver.
Significance of the study
Subthalamic-nucleus deep brain stimulator is a remarkable therapeutic-surgical technique that reduces tremors and restores motor function in patients with advanced Parkinson's disease both unilateral and bilateral STN-DBS, and for many disorders. The advances in stereotactic functional neurosurgical-techniques have fundamentally replaced ablative methods. Mahlon R. DeLong [19] formulated a new model for the brain's circuitry and exposed a fresh target for this illness. Benabid [21] devised an effective and reversible intervention that remedies neuronal misfiring in Parkinson's disease. However, there is no well-suited and available neuro-biomarker perfectly giving imperative information on the PD motoric and non-motoric features in subthalamic-nucleus and also globus pallidus interna. In this study, we account the results of neural-correlates of PD motor-symptoms in the territory of subthamic. Despite advances in magnetic resonance MRI particularly in connections with better spatio-temporal resolutions in latest 10 Tesla, the electrophysiological microrecording (MER) technique continues to be well suited for focusing on subthalamic detection and identification based on signatures (or “patterns”) in deep-brain stimulating procedure. [14], [15], [16], [17], [18] Therefore, we expect, the results will yield potentials for the construal explication of oscillatory-dynamics of subthalamic-nucleus and that these signatures or patterns which are confined very well by the intraoperative MER can be used as objective-tools for future technologies of neuro-modulation. The study also highlights the variability in spontaneous LFPs amongst the subjects and the neural-data
Deep Brain Stimulation Methodology
Deep brain stimulation has setup an outstanding management for Parkinson`s disease and for many diverse syndromes. Engineering advances in frontier technologies and stereotactic functional neurosurgical techniques have essentially replaced ablative surgeries for Parkinson diseased conditions. Parkinson`s disease has also non motoric manifestations (i.e., the fundamental cardinal features or symptoms) cognitive dysfunctions like cognitive-impairment (CI), cognitive-dementia (CD), autonomic-disorders, sleep, gait and gastrointestinal, and neuropsychiatric-signs. The scientists/ researchers are considering that the permutation of genetic, age and environmental-factors are concerned.
DBS is one therapeutic-surgical method for Parkinson`s which employs high-frequency electrical-pulses for STN-stimulus and connected-brain-areas. The implanted device sends electrical signals/pulses to the areas of the brain responsible for body movement that reduces tremors and restores motor functioning in subjects with advanced Parkinson`s disease. The microelectrodes are embedded deep in the brain and connected to a stimulating-device which can resemble a cardiac pace-maker; a neurostimulator uses electric-pulses control and/or regulate the brain. The DBS can abet reduce the features, i.e., PD symptoms of tremor, slowness of movement, stiffness and gait disability caused by the PD, resting-tremor (RT), and other movement dystonic (dystonia) disorder. The microelectrodes are used to record neuronal activity and macroelectrodes to record the local field potentials (LFPs). Both methods are effective for treating middle-and-late stage PD, amid progression in quality of life and motoric-features as diminishing the impediments of mounting quantity of drug use. [33]
In neural dynamics in particular neuromodulation, the action of DBS mechanisms are imprecise or uncertain. Albeit, accurate-electrode-implantation and stimulus programming may enhance motor-manifestations and allow for a reduction in antiparkinsonian medication-doses,[34], [35] DBS stimulus-parameters are set by subjective-evaluation-of-features. Physiological based quantitative-procedures are not employed to optimize the effectiveness of DBS for reducing-motor-symptoms and increase the motor functioning. [34], [35]
At the moment, the open-loop DBS systems do not use the sensors for recording and monitoring the brain condition. Hence, its parameters remain even despite of fluctuations in the disease-state. It is on a relative and virtual technological fester due to numerous factors which include a)limited Choice-of-signals/waveforms, b)capability to stimulate barely a single-location, c) Inept use of battery (energy is a constraint resource in all the battery systems) [19]. The merely allowable input is a periodic train-of quadrangle, square and rectangle waves/pulses[19] principally in a micro miniature part of the brain (say, a few millimeters [19]). While, some degree of customization for the development for the custom-built programmes/programming is acceptable (for instance, the parameters like stimulus-intensity, change of pulse-width, amplitude, duration and frequency of the square-wave pulse-train), resulting signals cannot generate an ample-range of responses preventively from targeted neural-system, thus limiting the patients-behavior. [19] This increased activity prevents neurons from modulating-activity in their neighboring structures creating an ‘information-lesion’ in the area. Further, in open-loop conventional DBS systems, a neurologist (usually a specialist in PD and movement disorders) tracks the patient`s clinical-state and program the device manually in heuristics manner (trial-and-error).
The long-term clinical studies have so far botched to show that high-frequency stimulation has been able to sluggish the progression of the disease. So called ‘earlystim’ clinical-protocols have only deep-rooted that it was nontoxic and risk-free and safe to stimulate subthalamic-nucleus well in advance than it was so far customary and established. At the investigation-level, both empirically/experimentally and pragmatically, Benabid [21], [23] and [19] had published that in MPTP-treated monkeys, high-frequency stimulation of the STN could protect neurons in the substantia-nigra pars-compacta and pars-reticulata (SNpc, pr). To test this ‘hypothesis’ in humans, one would need to perform STN stimulation at the early-stage, which is not merely justly sustainable given the surgical-risk, even if low, in patients who are still minimally impaired by the disease. [21]
Albeit, high frequency stimulation bequeath and bestow clinical -benefits (PD motor symptoms reversal) when the target area is highly pathological, it comes with significant costs:
Programming of the signal is very lengthy with manual intervention and no automatic procedure or online coding, thus no custom-built custom-made facility. Hence, cost-factor which is an additional burden to the patients.
No adaptation or version to patients’ requests
Surgically often battery-replacements and endemic control in close-proximity cognitive-loops amid likely and potentially probable poor side-effects.
The (DBS) electrode is connected to a wire, which sits under the skin and terminates at a neurostimulator (Figure 3)
The neurostimulator contains a battery operated energy-basis (as a source) that infuses electrical-current to the tip of the sensor (the electrode). The current impulses can be targeted in such a way that they are able to alter electrical-activity in diseased brain to alleviate some of the cardinal motor-symptoms in PD patients.
This study accounts the analysis of LFPs gathered during STN-DBS recording in PD, its clinical significances’ and corresponding connotations. Potential clinical applications of these data include the use of LFPs for the PD symptoms neuro-biomarker, to enhance the targeting of STN in the implantation of electrode, and enlightening the adaptive closed loop stimulating systems deep into the brain structures.
Methods
This is a retrospective study which was carried out at a tertiary care hospital with a dedicated movement disorder unit from South India. 12 subjects with diagnosis of PD as per United Kingdom Parkinson disease society brain bank (UKPDS-BB) criteria were included in this study. All the patients were willing to undergo the procedure and fulfilled the following criteria to be eligible for STN-DBS i.e., they had disease duration of 6 years or more, good response to levodopa, able to walk independently in drug “on” state and had normal cognition. All PD patients who were wheelchair or bed bound, had cognitive-dementia (CD)/cognitive-impairment (CI) phantasms similar to hallucinations or severe psychiatric disturbances nightmares were excluded from the study.
The DBS surgery was performed with two burr holes on the two sides (left and right hemisphere) based on the co-ordinates. Five channels that are introduced with the central-channel representing the MRI target while medial and lateral are placed in the x-axis coordinates while anterior and posterior are placed in the y-axis coordinates to envelop or to swathe an area of 5 mm diameter. Medtronic DBS leads#3387 envisaging annular contacts is embedded in the subthalamic-nucleus area (Figure 4). LFP’ recording was performed in all patients bilaterally (bilateral STN-DBS). Electrodes are gradually conceded during subthalamic-nuclei and signal acquisition was performed. STNs are detected with larger-noises and distortions with a larger-baseline and an asymmetrical-discharge by means of multiple-frequencies ([Figure 4]).
Local Field Potentials and their functional characterizations and inferences in Parkinson`s Disease
Local field potentials are usually fused signals. Basically frequency patterns of these waveforms are partitioned into four classes of frequency-bands, namely, delta-band, theta-band, alpha-band, beta-band, gamma-band, and high-frequency-band waveforms typically ranging delta-band(δ: 1Hz–3Hz), theta-band(θ: 4Hz–7Hz), alpha-band(α: 8Hz–13Hz), beta-band(β: 14Hz–30Hz), gamma-band(γ: 31Hz–200Hz), and high-frequency-band beyond 200Hz ([Table 1]). The amplitude of these waveforms increases as the frequency reduces in proportion to. All these frequencies are linked by a dissimilar echelon of stimulation of the cerebral-cortex.
| Bands-frequencies | Range (in Hertz) |
| δ-waves | 0Hertz – 3Hertz |
| θ-waves | 4Hertz – 7Hertz |
| α-waves | 8Hertz – 12Hertz |
| β-waves | 13 Hertz – 30 Hertz |
| γ-waves | 31 Hertz – 200 Hertz |
| High-frequency waves above 200 Hertz – 500Hertz |
Fundamentally nothing is pathological about a given oscillatory-frequency-range (OFR). However, spectrum (the power-spectral-density - PSD spectrum) moderately represents a complex band of neuronal-activity – the significance of which depends on its spatio-functional milieu. This complexity necessitates a thorough estimation of LFPs for every DBS signal and target-location of every channel-electrode. The direct amplitude and power of the LFP-signal acquisitions are implicit and are assumed to represent the degree of synchronization between neurons surrounding the electrode tip[36], [37] A transient increase in power, in response to a specific-signal sign, is often referred to as an event-related-synchronization (ERS), while a transient decrease in power is expressed event-related-desynchronization (ERD).[6] Both ERD and ERS are typically computed by averaging the power across-temporal-segments and comparing this standard to a reference eon or an epoch [7]. In electrocorticography experiments, observations indicated that these cortical ERDs in α and β-bands were coupled with an ERS in the γ-frequency-band. Consequently, an ERS can also be activating. Thus, as a result, the interpretation of ERD and ERS phenomena is likely to depend on the tissue of interest and other elements of the f-band. [8]
The local field potentials had given important scientific insights in to Parkinson disease in particular with β-band oscillations. This interest branching from observations in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primate-model.[6] With LFPs, the β-fluctuations associate positively with rigidity and also bradykinesia motoric-features. The consequential loss of dopaminergic neurons in the SNpc, the feature of trademark of PD, is differentiated by the onset of Parkinson’s motor-features like Bradykinesia and rigidity in these MPTP-treated monkeys.[9], [10], [11] Signal acquisitions from single-neuron single-unit-activity recordings from the subthalamus and also globus-pallidus in these monkeys have acknowledged an augmented stimulus-firing-rate accompanied with synchronous fluctuatory bursting-activity which was not found in normal-controls.[12], [13] The augmented flucuatory-activity examined in MPTP-treated monkeys was consequently set up in the β-band frequency of local-field-potentials acquired from the subthalamic-nuclei of human Parkinson`s underwent-surgery. Attractively, the existence of this β-band is removed by dopamine-management ([Figure 5]),[14], [15], [16], [17], [18] and the degree of improvement in Bradykinesia and rigidity following dopamine-management has been signified to correlate with the magnitude of β-band suppression.[19], [21], [22]
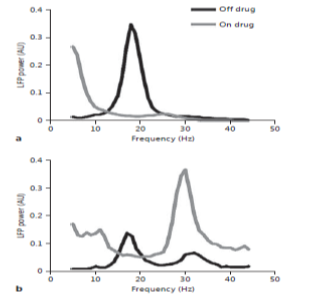
Kuhn et al.[37] equally observed that undue synchronous β-activity can be induced in patients undergoing GPi-targeted DBS for dystonic-movement by using the dopamine antagonist tetrabenazine. This evidence suggests that highly synchronous β-activity is caused by dopamine depletion. Interestingly, initial studies did not find a correlation between unprocessed β-power and severity of Bradykinesia/rigidity in patients withdrawn from dopaminergic medication.[38], [39], [40], [41] However, the absence of influence of local-field-potentials standardization in these investigations might shrink the capability to identify an important connection among β-fluctuations and motor mutilations. For instance, β-influence is familiar to diverge spatially over minute detachments. Hence, delicate changes and thus disparities in electrode location virtual to the target might diminish the connection amid the mutilation of motor and unrefined-raw β-influence. Very recently, supplementary revisions have used circuitous approaches to standardize synchronous β-activity among subjects and have acknowledged a momentous connection amid β-fluctuations and motoric-features like Bradykinesia, postural-instability and rigidity. Thus, the control approaches consist of the implications of β-band signals or waveforms of local field’s intricacy plus the degree of time-coherence among the fingering with neighboring leads of deep brain stimulations.
Similar to the effect of dopamine, STN-targeted DBS causes suppression in the β-frequency fluctuations and the degree of improvement in rigidity and bradykinesia correlate with the magnitude of β-suppression (measured immediately after stimulus discontinuation). However, this has not been a universal finding. A limitation of these studies is that the authors were not able to record LFP during stimulation. To address this, Rossi et al. [27] developed a stimulus suppressor capable of recording ripple-free β-band LFPs whilst asynchronously/concurrently stimulating the subthalamic-nuclei. Using this technique, these authors found that stimulation through the DBS electrode suppressed β-synchronization in patients who were off dopaminergic-drug-medication but not while they were on medication. Therefore, while DBS and dopaminergic medication both diminish β-LFP power, the effect of dopaminergic medication appears to be stronger ([Figure 6]).
From the indications, [15], [42], [27], [24] the ‘hypothesis’ is also sustaining that β-activity is anti-kinetic associating β-influences with the intended connection. The β-event-related-desynchronization was examined during the latency and slowly progressing the event-related-synchronization through progression terminated. Besides, while patients were cared not to turn ensuing to grounding progressively, a noteworthy harmonization was examined in the β-band.[19], [21], [22], [25] These observations support the ‘hypothesis’ that β-band activity is anti-kinetic. While β-band LFP activity has received intensive scrutiny in recent years, it is important to keep in mind that the limits of the field-potential frequency bands are somewhat random or sporadic. For instance, there is evidence that low β-activity (12Hz–20Hz) may derive from a different underlying physiological process than high β-activity (20Hz–30Hz). This is supported by the observation that oscillatory-power restraint in retort to dopaminergic-drug-medication or prescription is greater in the low β-frequencies differentiated to high β-frequencies. However, an understanding of how these different β-associated-bands relate to the underlying pathophysiology of PD remains enigmatic and mysterious.
Higher-Frequency Fluctuations
The θ-band, γ-band, and high-frequency bands influence have been found to enhance the dopaminergic-drug-medication in disparity to the β-band-activity. However, as and when patients are not on levodopa, the γ-band-activity amplifies bilaterally and actively. This augment in γ-band-activity becomes lateralized to the brain`s contra lateral to the active-motion in the on-state. Hence, the implications recommended are that γ-band-activity might advance the usual voluntary-movement. Also, lateralization of the event related synchronization implies that dopaminergic-drug-medication re-establishes this activity to an additional and typical patterns physiologically. Therefore, high-frequency-fluctuations (HFF) more than 200Hz have been identified in the subthalamic-nuclei of PD-subjects going through DBS for other movement disorders such as dystonic-writer`s and musician`s cramp and essential movement disorder tremor that are measured hyper-kinetic MDs.[36] In addition, dopaminergic-drug-medication may develop high-frequency-fluctuations. [43], [29], [37]. Particularly, an augment in fluctuatory-influence may vary from 300Hz to 350Hz frequency has been monitored on 250Hz fluctuations. Furthermore, the significance of the high-frequency-fluctuations are highlighted by examining the combination of β-fluctuations and high-frequency-fluctuations among the PD-patients pathophysiologically. Hence, the high-frequency fluctuation of β-association has been radically satisfied and subsequently the treatment of dopaminergic-drug-medication in subjects with lesser disease has to be distinguished. Thus, the gamma-band and the high-frequency band-activities are usually measured in order to cooperate and lead for major responsibility as a pro functioning of kinetic during the Parkinson`s disease cardinal-motor-features.
Dyskinesia
Studies in other movement disorders, such as Dystonia,[38] have shown that the long term dopaminergic-drug-medication with levodopa (L-Dopa) in Parkinson`s has lead to the growth of dyskinesia. The PD subjects who have undergone DBS surgery by implanting electrodes particularly in the globus-pallidus interna and subthalamic-nuclei, the levodopa induced dyskinesia data was associated with influence of local field potentials. Thus, the acquired biomarkers (biosignals) from these neurons confirmed that the desynchronization events in the assortment of b-frequency are coupled with the dyskinesia-states considerably. [39]
Resting-tremor
Majority of the investigations have explained that the Parkinson`s resting tremor did not connect with frequency-fluctuations. Yet, epochs in which significant rest tremor is exhibit and have been correlated with better-influence in the lower γ-band-frequency (35Hz–55Hz) which is typically sighted like pro-kinetic. In addition, the fluctuations are approximately frequency-tremor (typically in the band of 4.6Hz to 5.8Hz) and binary-frequency-tremor (~ 10-5Hz) associate with tremor-related electromyography-signal-activity. These potential fluctuations plus the coherence with hand-shake emerge to be spatially restricted in groups within in the terrain and zone of the subthalamic-nuclei, and, to a lesser extent, the incerta-zona. Attractively, the location of the groups of the coherence-electromyography inside the subthalamic-nucleus fluctuates among the inactive and postural tremors associated with PD. Furthermore, tremor-related activity is more and can be easily identified with the acquired local-fields from the subthalamic-nuclei than from the globus-pallidus-interna. Thus, among the local-field-potential fluctuations and tremor, the coherence is twice at the tremor-frequency and might characterize a real connection physiologically. On the other hand, while contrasting electromyography and local fields that are tremor linked, consistency between tremor-dominant and Brady kinesia associated-groups, the differences in consistency (or rationality) within the frequency-tremor assortment among the associated-groups of Parkinson`s emerged to be independent of differences within the binary-tremor-assortment. Hence, this inference holds the ‘hypothesis’ that local fields and binary-frequency-tremors are significant physiologically.
Postural Instability and gait difficulty
DBS is slightly futile in the management of postural-instability and gait difficulties. [4] Gait-difficulty and postural-features like instability do not react in response to modern dopamine management, signifying that they are forbidden by a neural-pathway which is separable from the pathway of dopamine-dependence. Studies in Nonhuman primate have showed that the pedunculo-pontine-nucleus (PPN) is implicated in the induction and maintenance of locomotion. Building on this work, stimulation of the PPN has also been investigated in the treatment of PD.
The local-field-potentials acquired from the leads of DBS implanted in the pedunculo-pontine-nucleus (PPN) in PD patients have confirmed that the influential fluctuation within 7.5Hz to 11.5Hz is improved with the dopaminergic-drug-medical management. Also, the L-dopa management was creating to stimulate synchronization among local field potentials in the range of 7.5Hz–11.5Hz and concurrently acquired electroencephalography-activity. In pedunculo-pontine-nucleus signal gatherings of Parkinson`s during off-state, β-band-frequency crest had been descripted.
Essential-tremor and local fields
The stimulations aimed at the intermediate-ventral-nuclei of the thalamus for the modern-management of tremor was the first application of deep brain stimulation which was approved by the United States of America Food and Drug Administration (FDA, a federal agency of US Department of Health and Human Services, and one of the US federal executive department), and it remnants[8] the next commonest implication of deep brain stimulation. Endeavors towards the association of local field potentials amid tremor had been convoluted by the microthalamotomy/micro-lesion upshot [8] which was frequently examined throughout the surgery for the management of essential-tremor. In spite of this confront the 8.5Hz to 27Hz frequency fluctuations post operatively gathered using DBS leads are coherent [8] with the frequency-tremor significantly with noninvasive electromyography of the first dorsal interoseous muscles. Thus, the study revealed that the local field potentials give good amount of the analysis about the spatial heterogeneity of the thalamus, which might assist with the localization during the DBS-surgery, and also about disparities in the spatial synchronization of local field potentials, that may be associated with the pathology of essential-tremor.
Dystonic movement and field potentials
Dystonia” movement-disorder was first used by Oppenheim (1911) [20] to elucidate a disorder causing variable muscle-tone and periodic and persistent muscle-spasm. Currently dystonia is referred to as “a neurological syndrome fundamentally characterized by involuntary, sustained, patterned, and often repetitive muscle co-contractions of opposing-muscles, causing twisting-movements or abnormal-postures correlated with significant twinge and disability ”. [44], [45], [46] The genetically disordered (genotype) symptoms could be indiscriminated structurally due to damage of the brain`s central nervous system, genetic-mutation, or other disease-states. In 2003, the US FDA approved DBS for the management of segmental and generalized-dystonia on humanitarian device exception and exclusion-condition. However, even though the US FDA endorsement permitted for aiming at both globus-pallidus and subthalamic-nucleus the globus-pallidus has turn out to be the option of deep brain stimulation especially in “dystonia-neurological-syndrome”.
In many experimental observations, the spectral and power spectral-density and the frequency-spectrum of local-field potential waveforms acquired with microelectrodes implanted in globus-pallidus-interna for the management of dystonia has been established. These observations have constantly descripted and moderately and virtually an elevated-influence in the band of 3Hz-12Hz frequency selection evaluated to supplementary frequency-bands. The local field potentials acquired from globus-pallidus had been exposed to harmonized by parallelly acquired spike-activity in disparity to the global pallidal neurons. Whereas, the frequencies of 4Hz to 10Hz, 11Hz to30Hz and 65Hz to 85Hz are considerably associating with the sternocleidomastoid muscle-signal in diseased subjects with cervical-dystonia and also in myoclonus-dystonic-subjects. In parity with myoclonus-dystonia, the local field potentials in the range of 3Hz to 15Hz are coherent-feature with non-invasive electromyogram commotion coupled by exaggerated muscle groups. Therefore, this coherence was discovered to be robust throughout the grounding plus implementation of extensor aspect of fore arm wrist-extension tasks and also flexor aspect of wrist-flexion tasks. Amusingly, the reaction to the intentional association diagonally the band of local fields acquired from the pallidal neurons in the dystonic Writer`s cramp and also in the Musician`s cramp patients is analogous to the reaction examined and the implications drawn in subthalamic-nucleus by analyzing the local field potential signals (acquired from STN) in PD-subjects. Therefore, in the dystonic-patho-physiology these findings are suggesting that the fluctuations with low-frequency are implicated.
Local field potential recordings
Location of the target
It is well experienced that the identification of the target-location accurately and then pinpointing the lead for implanting the electrode perfectly inside the structure of interest (few millimeters, say 2mm to 6mm) in constituting a maximum; as great or as large as possible maximal-diameter and which was situated various centimeters from the cortical-entry site is a hazardous and recording the LFPs effectively for determining the subthalamic-nuclei deep brain-stimulation in Parkinson disease, and also for studying the effectiveness of LFP recordings in deciding the final tract for placing DBS electrode during bilateral STN DBS is complex. Furthermore, the structural variation of inter diseased patient and inadequate magnetic resonance image resolution make it mandatory for the neurosurgeons to carry out electr-neuro-physiological signals with microelectrode recording (based on MER signal patterns or signatures). With microelectrodes recordings, the neurologists have to depend on the parameters of single-unit singe-neuron-activity mainly to deduce the electrode positioning-point in brain. Hence, the parameters are vulnerable to scientific research technical-configuration, such as, fluctuations due to impedance, and also physiological cerebrospinal fluid and blood fluctuations. Therefore, the skewed nature of inferring signals of MER may direct to unpredictability in connecting with single-neuron/single-unit activity through brain location within the zone. Because local fields replicate a collective electrical-stimulations-activity in some of the neural-tissues, hence, due to this discrepancy, the local field fields are resistant to physiological fluctuations very fundamentally. Moreover, as these potentials are less-variable constraints diagonally, the diagnostic utility-tools which are designed to associate local fields’ activity with the depth-of the electrode might voluntarily distinguish the practicing-performers and also allow data-consistency, inferences and explanations further. These local-fields have been using since long to demarcate the subthalamic-nuclei perimeters. Principally, β-influence fluctuation. In fact, stimulus of the dorso-lateral subthalamic findings in finest symptomatic development through least stimulus-connected side-effects contrasted to ventromedial-stimulus. Stimulus of dorsolateral subthalmus results in fine symptom development through least side effects in contrast to ventromedial stimulus
The field potentials influence in the β-band-frequencies.[5] This has been shown to connect with the distribution of features physically associated regions of subthalamic-nucleus and with single-unit of single-neuron activity acquired from subthalamic-nucleus asynchronously. [5] In a study, while estimating coherence among tremor-related electromyography plus local-fields, the spatio-temporal allocation of coherence-groups coupled through the point-of-contact which was then selected for constant-stimulus considerably [47] and the electromyogram activity was consistent among field-potential in shaking-hand and in the binary-tremor-frequencies. This supports that the activity (the fluctuatory-activity) acquired from basal-ganglia(BG) may sustain stimulating electrode with deep brain stimulations. Considering the above analysis largely, the local field potentials have the benefit of regularizing version of subthalamic-nuclei associated segmental peripheries than using with the existing microelectrodes. The offline local fields potentials analysis my botcher wrong and hence the real-time online computations is worth and more scientific objective evidence for implanting the DBS leads for chronic stimulation.
Another important issue in the current open-loop deep brain stimulation devices is “programming” which is a set of instructions defined manually by the end user who is an expert neurologist. In the open-loop DBS systems, the programming is very cumbersome because of lengthy pictorial and a sequence of instructional coding method in which each of the four contacts are examined independently. This takes a lot of time which is very cumbersome and also consuming the charging device energy (“the battery”) considerably. The end-user makes use of virtues and demerits such as side-effects scientifically and subjectively via the PD subject self-report and finally chooses best choices and the best optimal-settings for stimulations chronically (“chronic-stimulus”) which is arduous and lengthy procedure and also a burdening issue to the neurologist and feeling uncomfortable by the patient. But, present DBS leads (macro-electrodes) are increased with increasing-complexity of programming. One of the examples[48] is a 64-lead electrode, which could allow for guiding of voltage which was away from the showing of the current. Martens et al. [48] have designed a 64-contact DBS lead that may let for the conducting of voltage missing from structures like inner-shell – resulting side-effects by stimulus as an auxiliary and undesirable effect of the charging. Therefore, by employing various tiny chips (say, micro-size or nano-size or even further smallest pica-size) arranged approximately the perimeter of a firm-sustain as a replacement of the cross-sectional links employed scheduled modern-chips. Even though, the existence of sixty-four DBS leads shall make existing coding and programming-techniques idealistic, albeit, the new diagnostic routines establishing or founding on field-potentials have been built to evaluate spatial-contact as well as effectiveness of stimulus through deep brain stimulator. There are several studies on local field potentials for enhancing deep brain stimulator outcomes with LFPs diagnostically and/or clinically in setting up of implantation of leads into the subthalamic-nuclei and that can be found in. [27] Current studies on local field potentials[2], [3], [4], [5] advocating that deep brain stimulation coding principles and techniques which can be enhanced by the mathematically framed models and computer simulated parameters of DBS and by implications and inferences of DBS leads.
One direct clinical application relates to the chronic hyper-synchrony evidenced in the basal ganglia of Parkinson’s patients: better clinical outcomes correlate with the degree of β-hyper-synchrony. Analysis of LFPs through the macro electrode could be used to set optimal-clinical stimulus-parameters. Interestingly, those contacts associated with optimal-stimulus efficiency significantly correlate with contacts exhibiting maximal γ and β-bands influence. Moreover, stimulus (the chronic-stimulus) through contacts which are further from the apparent source of β-commotion is associated with a poorer response to stimulus. Thus, LFPs recorded from implanted DBS electrodes might prove useful in improving and automating the programming process. One specific advantage of a DBS electrode design that has denser, smaller diameter contacts would be the selective distribution of electrical activity patterns (‘steering current’). Furthermore, activity patterns could be based on LFP information, which may reduce the overall stimulus output, thus increasing battery life.
Adaptive Deep Brain Stimulators
Currently, the existing adaptive open-loop DBS-systems have the lacuna, such as, unable to scrutinize both the motoric and nono-motoric symptoms of PD patients and consequently parameters not accustomed or tuned, the coding-personnel has to adjust the stimulus-parameters (voltage, current, pulse-width, frequency, stimulus-intensity and contact-selection) can only be modified by coding-personnel using an external-pointer (the truncheon) which placed over the patient’s pulse generators implanted in them. This cumbersome-procedure requires patients to visit the staffed personnel with personnel who have expertise in coding the program implanted DBS devices, thereby inconveniencing patients and lumbering these clinical-centers with repetitive appointments. Furthermore, without a sensing-component to the existing-DBS-devices, there is no feedback and control mechanism to let the DBS-device to be turned-off during patient sleeping, whilst Parkinson’s symptoms disappear, or to be ramped-up when symptoms increase amid medication doses. Therefore, there is much interest in developing a closed-loop therapy in which a relevant neural marker, such as local field potential fluctuations, gives feedback that directs the modulation-of-stimulus parameters in real time. Rosin, et al. [49] demonstrated the superior-function of closed-loop/adaptive DBS, which automatically adjusts the stimulating parameters, to alleviate the motoric-features of PD. Rosin investigated the feasibility of such a closed-loop stimulating device in an MPTP primate model of PD. Single-unit of single-neuron recordings from the primary motor cortex (M1) and the globus-pallidus interna were used to direct the stimulation of the GPi. Specifically, the detection of a spike in either the GPi or the M1 triggered a crouch-potential-train stimulus (circa ~ 7 pulses at a frequency of 130Hz, and 80-ms duration). This form of closed loop stimulation proved superior to continuous, GPi-targeted stimulation in suppressing pallidal-neural-spike and fluctuating-activity. Furthermore, closed-loop stimulation was associated with a greater reduction in akinesia compared to continuous stimulation.
At present, the cardiac-device-battery (pacemaker) life of non-rechargeable deep brain stimulator pulse-generator is approximately, circa ~ minimum 3 to maximum 5 years, depending on the stimulus parameters used. Also, day by day the need for this device implantation in PD patients receiving the DBS interest more and more and increasing in proportion. The implanted pulse-generator replacement surgical-procedure typically involves twenty minutes duration accomplished performed under monitored anesthesia care. Replacement surgeries are associated with a small but significant risk of infection and damage to the existing device. Thus, strategies to increase the battery life of DBS devices are important, considering the number of battery replacement surgeries required over the lifetime of, for instance, a 40-yearold patient with essential-tremor. If a closed-loop device was capable of identifying when a patient was asleep, stimulation could be turned off during this period, thus increasing the battery life of the device. In fact, β-frequency band field-potential influence has been reported to be significantly lower during stages 2 and 4 of sleep compared to when patients are awake. It is not practical to require PD patients to self-regulate their implantable pulse generator power for sleeping and waking, as a significant number of patients with PD often experience neurogenic bladder dysfunction, which is associated with a greater frequency of micturition. Intentional tremor, a primary sequela of PD, would make turning the implantable pulse generator back on (for bladder evacuation or upon waking) difficult if not impossible. Additionally, different motor symptoms may be determined to have unique LFP profiles, which could be ameliorated with a specific combination of stimulation parameters or by switching active contacts. For instance, disparities in stimulation efficacy for Bradykinesia have been observed to depend on the site of stimulation within the STN. Switching off electrodes placed in the lateral STN resulted in a rapid return of Bradykinesia; however, a more medial placement resulted in a slower return of Bradykinesia. Such stimulation device improvements could increase the time between battery changes.
Another consideration for developing a closed-loop DBS device is the integrity of the electrode-brain interface. Postmortem histo-pathological studies have found the DBS electrode in the brain to be encapsulated by a thin, GFAP-positive capsule years after initial implantation. Moreover, a recent study showed that in patients with PD, the LFP power in the β-frequency band was significantly lower 3 to 7 years after the initial DBS implant compared to power recorded at the time of DBS surgery. In contrast, the magnitude of the movement- related desynchronization in the β-band was preserved and detectable over time Therefore, despite the decrease in β-influence over time, the ERD preservation supports the feasibility of using β--band activity to inform a closed-loop device.
Coherence
Coherence between the tremor-dominant and fluctuations of field-potentials at twice the frequency of tremor possibly will signify a real physiological-correlation, ripples-noise and distortion or a combination of both.[45] However, when comparing tremor-related EMG-LFP coherence between tremor dominant and Bradykinetic PD symptoms, the disparities in coherence in the single tremor frequency range between PD subtypes appeared to be independent of disparities in the binary-frequency-tremor range.[8] This supports the ‘hypothesis’ that field potentials binary-frequency-tremor is significant electro-neuro-physiologically.
Coherence model estimation
Let, ‘x’ and ‘y’ be two signals of same-length (generally, input/output). Coherence is a function of frequencies which points how well the input signal ‘x’ fits to corresponding output signal ‘y’ at each time-point. This model is originally designed by [50]
The coherence is mathematically framed or modeled as, [50]
Let Px x and Py y be estimated power spectral densities of x and y, and let Px y be the cross power-spectral-density (PSD) estimators of ‘x’ and ‘y’.
Generally, x and y are separated into identical or similar number-of-blocks, say eight blocks of same size. Periodograms of each of these blocks are computed and are averaged out to get better periodogram estimates. From these periodograms the power spectral-densities Px x, and Py y and Px y are evaluated.
The (magnitude squared) coherence function Cx y is estimated and is computed as[50], [51], [52]
Pxy.2.Pxx.*Pyy .................................................... (1)
where, in the equation (1). * and. / stand for term by term multiplication and term by term division of the sequences concerned.
Thus, for each frequency, magnitude square of the Px y at that frequency is divided by the products of Px x and Py y at the same frequency.
This is like the usual correlation between two sequences and is a measure of agreement (i.e., degree of linear relationship) between the corresponding terms in the two sequences
The disparity between a simple correlation value and the coherence function is that, at each frequency, a correlation like measure is computed and the coherence function gives this (squared) coherence between the two signals at each of the frequencies.
Notation
As an example for the above given notations, consider
Px y: 2, 1, 4, 6, 3, 5 be one sequence, .................... (2)
Px x: 5, 2, 3, 4, 1, 6 is also one sequence ................. (3)
Py y: 3, 5, 2, 8, 6, 9 is also another sequence, .........(4)
Subsequently
Pxy.2=4, 1, 16, 36, 9, 25.................................. (5)
Px x .* Py y = [15, 10, 6, 32, 6, 54] ............................(6)
Cxy=Pxy.2Pxx.Pyy ...........................................................(7)
Cxy=45,110,166,3632,96,2554 .....................................(8)
In our computation, coherence band will have two levels 0 and 1. For decimal values we have taken 0.75 and above for considering the coherence value as a valid. For instance, a coherence value at a given frequency being nearly 1 means: at that frequency, the two signal components are resembled, while, a low (near to 0) indicates that the signal components at that frequency do not have any resemblance and hence did not show any association or connection. The frequency resolution of the spectral analysis signals was 0.95Hz, and coherence is considered to be highly significant if it is exceeded the degree of confidence level 93%. [23], [53], [51], [52] Using the equation (1) and modeled equation (2) the coherence was computed and the following results were obtained.
In [Figure 7], the coherence computed signals similarities between 1, 2 and 2, 1 ([Figure 7]) is showing symmetry by the side of the sloping. Hence, the [Figure 7] (frequency of the signal with coherence) in row 1 and column 3 is same as that in row 3 and column 1.
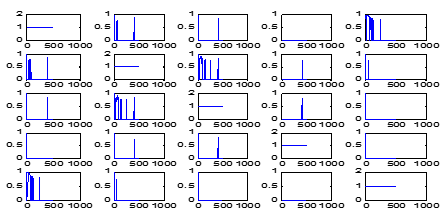
Adding to this, in Figure 7 coherence computation deciding and depicting only the frequencies at which coherence has the value > 0.75. Therefore, the first-row fourth-column panel showing the LFP-STN pair of neurons 1 and 2 is blank. Subsequently, the coherence panel in Figure 8, second-row first-column panel, has no frequency, however, the coherence > 0.75, whilst, the next panel has quite a few-frequencies which are having coherence < 0.75. Analogous to the case with LFP-STN pair 2 and 3 is consequent to the second row and third column of Figure 7, and third row first column of [Figure 8].
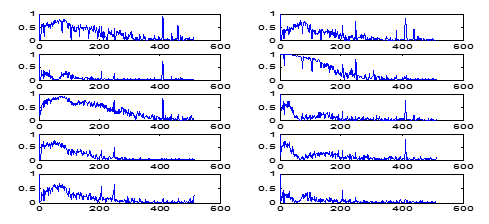
Mathematically the computation part is derived as follows:
Variance
Variance is the average of the squared deviations of each data point from the mean value. For a population of size N, the variance sigma square, is computed as, [19], [50]
σ2=1N[∑i=1Nxi-μ-2] .........................................(9)
Similarly for a sample size it is denoted by s square and is evaluated as,
S2=∑i=1n(xi-x-)2(n-1) ..................................................(10)
The mean μ- [σ2] and the variance Var [σ2] of σ2 are computed as,
Var [σ2] = μ- [(σ2 − σ¯2)2] = Var[ε] .................(11)
Because x is written as the convolution of σ and white Gaussian-noise W passed through H, the conditional distribution of x given σ2 is Gaussian distribution with a mean of zero and a variance of σ2:
Pxσ2=1√2πσ2exp-x22σ2..................................... (12)
Standard deviation
The standard-deviation (or SD) of a sample is the square root of the variance which is measured as [19],[50],
S=S2=∑i=1n(xi-x-)2(n-1) ......................................(13)
consequently, the sigma
‘σ’ = σ 2 ............................................................(14)
is SD of the population.
Root mean square
The root-mean square (RMS) or root mean square deviation (RMSD) or root-mean-square error (RMSE) (or sometimes root-mean-squared error) factor mostly used in engineering and medicine for a statistical significance. It is often used for computing the disparities among the values (sample or population values) predicted by a model or an estimator and the values observed. In our computation the sample size is N (=12).
To compute the RMS of a given population N, in our case it is (N=12), one has to square the given population in the N and then determine the arithmetic-mean of the squares. Finally, take the square root of the findings.
It is measured as,
RMS=a12+a22+…an2n=a12+a22+…an2...... (15)
or, discretely as, ∑i=1nai2n .................................. (16)
In our 12 population computation, the RMS for the brain left hemisphere during DBS “ON” is 1.1±0.8 and during “OFF” it is 5.9±11.9 ([Table 2]). Likewise, RMS for the brain right hemisphere during DBS “ON” is 0.9±0.3 and during “OFF” it is 2.6±4.7 ([Table 2]).
| Features of LFP PD Subjects | |
| DBS with “OFF” Waveforms | with DBS “ON” |
| RMS with BLH 1.1±0.8 | 5.9±11.9 |
| RMS with BRH 0.9±0.3 | 2.6±4.7 |
| Coherence BLH 1.5±0.7 | 2.3±1.6 |
| Coherence BRH 1.4±0.8 | 1.6±1.5 |
| Entropy BLH 1.4±0.7 | 2.3±1.6 |
| Entropy BRH 1.3±0.7 | 1.5±1.6 |
Entropy
To quantify or compute the local field potential signals (LFP signals), the thermodynamic entropy is used. It is a measure of the fraction of the internal energy of a neuron which is not available to function. In neurons impulse progression, such as the flow of neuron from one state to another state (i.e., neuron sending impulses from one state to another state typically referred to as “divergency” and similarly receiving impulses from other state neurons is usually referred to as “convergency” that is from normal to abnormal and vice-versa. It is said that entropy is increases at all times. [2]
To establish the functional nonlinear multivariate (Taylor`s series) LFP signals (say F) in a deep brain stimulation correlation or relation
r(t)=Fs(t)..........................................................(17),
to discretize prior times:
s(t)=(s(t-Δt), .., s(t-LΔt))= (s1, s2, s3.., sL) ..(18)
Consider the only current-response:
(r) = r(0), ................................................................(19)
F is a multivariate LFP signals for r in terms of (s1, …, sL). So, to compute low-order coefficients, assume others are 0. Hence, the maximum-entropy is computed distributed as
K-exp-cr-Fs1,...sL2.............................(20)
In multivariate analysis, the mean and covariance’s controlled or conditioned as:
K- exp(x-mTC(x-m)} .............................................(21)
and in independent variable or analysis, with marginal’s
P (x), Q(y): P(x)Q(y)..............................................(22)
In this study, the entropy method is of non-linear-dynamics (signals with different phase amplitudes and with different frequencies) which computes negative (-Ve) natural-logarithm of the conditional-probability that two-sequences in a time series/time-domain which are analogous for ‘m’ number of points are analogous for m+1 points [54]. In our computation, we obtained the entropy value which showed the tremor-complexity during DBS “ON” for the brain right-hemisphere (left side: 1.4±0.7) and “OFF” states (2.3±1.6), and for the right hemisphere the tremor complexity during DBS “ON” is (1.3±0.7) and during “OFF” state in left-hemisphere it is 1.5±1.6 ([Table 2]). So, if we observe, in our computation, carefully, as we already mentioned that entropy is increases at all times [2], and so the entropy is increased in either case. This also (the results) indicating that the PD-patients cardinal motor-symptoms were effectively reduced with DBS.
Discussion, Conclusions and future directions
Deep brain stimulator (DBS) is an innovative frontier surgical therapeutic technological method for almost maximally uprooting symptoms of Parkinson’s disease (PD) and other movement disorders (MDs) as well. DBS provides a unique opportunity to study the electrical oscillatory neural-activity of various sub-cortical-structures in PD-MDs subjects and epileptic-seizures too. However, the electrically stimulating local field potentials are concerned during subthalamic-nuclei (STN) recording. The acquisition of extracellular activity of irregular patterns of STN and globus pallidus (GP) brain activity typically acquired from a population of neurons detected as local field potentials has discard luminosity on the pathophysiology and seizes the latent to pilot to elegance in modern management. A recent study [55] gives us very remarkable approach into pathophysiology if the stimulus is through deep brain stimulator to the subthalamic-nucleus (for Parkinson disease) and also pallidal neurons (for the dystonia). Benabid in his long experiments stressed that constant stimulus won’t be damaging or injuring or denting the neurons. Therefore, stimulus through DBS has no side-effects.
The above study underlines that constant stimulus reactivates the neural-cells and thwarts apoptosis. Thus, stimulus through DBS has prospective-benefits of varying the disease-process. This has been observed experimentally and clinically in our patient population, and patients those underwent stimulus through deep brain stimulator had a longer endurance plus improved quality-of-life contrasted to their accomplices who had remedial medical drug treatment in only. The field potentials gathered from deep brain stimulating electrodes in PD patients have given neuroscientists with a novel method for understanding, and potentially refining treatments for movement disorders. Correlating aspects of the LFP frequency spectrum with clinical symptoms has provided new insights into the pathophysiology of these disorders, and mounting evidence suggests that LFPs will be useful in improving current therapies in this arena. In particular, LFP fluctuations have proven to be useful in localizing DBS surgical targets. LFPs may ultimately be able to inform a closed-loop DBS device that is responsive to individual patient symptoms in real time.
Studies of the behavior of STN neural recording and the underlying physiological functions of human motor system are of significant importance to both clinicians and basic researchers. Analysis of electrical activity of neurons using local field potentials (LFPs) have demonstrated alterations of inter spike intervals, specific firing patterns (signatures), and oscillatory activities of neuronal cells in PD and LFP-STN contain electrophysiological footprints of motor sub types of Parkinson`s disease [2]. Microelectrode recording (MER) or microelectrode signals recording of local field potentials with subthalamic-nuclei deep brain stimulation is most useful for interpreting Parkinson diseases (PD) signal analysis acquiescent to elucidation are fetching ever more germane or pertinent. These signals are supposed to emulate STN neurons action-potential movement and, these potential frequency modulations are coupled to spiking-events.
The field potentials have provided basic neuroscience – neurology and neurosurgery researchers and clinicians amid a innovative method for perceptive, and potentially demonstrating and improving management for Parkinson`s disease and movement disorders. Correlating aspects of the LFP frequency spectrum with clinical symptoms has provided new insights into the pathophysiology of these disorders, and escalating data implies that LFPs will be valuable in the progression of contemporary modern management in movement disorders. In meticulous, LFP fluctuations have proven to be useful in confining DBS surgical targets. Eventually, field potentials possibly will be capable and proficient to enlighten the adaptive closed-loop DBS devices that are responsive to individual patient symptoms in real time. The LPF biomarkers are imperative in designing the adaptive closed-loop and next generation DBS systems. The new DBS systems are expected to be automatically programmable and also compatible with LFP signal variations, flexible to stimulation types and patterns, to give better benefits. With the LFP-biomarkers there is a less chance of increasing the malfunctions and hence it may guarantee the robustness of adaptive operation of DBS devices and based on multiple LFP-biomarkers it is easy for operation. The engineers need to provide a balance and trade-offs among the device features by considering the effective medical and thus clinical diagnosis, and the technological costs of the DBS devices.
Future directions
There is evidence that non-Parkinsonian neural activity is asymmetrical, much lower in frequency. Sarma, et al [9] attempted to restore-neural-activity and address the shortcomings of high-frequency deep brain stimulation (HF-DBS) using alternative low-frequency and asymmetrical stimulation-patterns, but the clinical-outcomes reported are very sparse.[9], [10], [11] These poor results stem from both technology limitations of fixed-stimulation in a single location and lack of understanding of the neural circuit being stimulated. DBS strategies (different and/or multiple sites and patterns) for PD could be evaluated if we have a deeper understanding of the dynamic interactions between nuclei in the cortico-basal-thalamic loop in normal and in PD, with and without DBS.
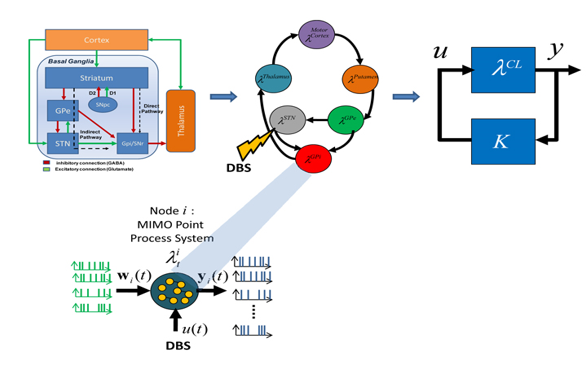
David, et al.[9] employed Auto Regression (AR) modeling for analyzing the LFPs through control theory and systems approach. The technique is employed extensively in different fields of engineering and medicine (for effective electro-neuro medical diagnostics) for several ranges and co-ranges and domains and co-domains in statistical signal processing. Cassidy, et al.[56], [52] employed a variation of the method to that of David et al, [9] however opt a singular (dissimilar) path for investigation.[52] David et.al [9] employed dynamic-multivariate-auto-regressive (dMAR) models to investigate the non-stationary coherence among LFP signals in the STN and GPi, and with electroencephalography (EEG) signals. However, the principal components analysis (PCA) method for analyzing LFPs is not yet employed so far. In this direction, some work has been done (refer our previous paper [17]) by us. We are now developing a novel system modeling framework for studying the dynamics of the BG-cortical loop. In order to develop this modeling framework, we will exploit a unique experimental set up (microelectrode single-unit and local field potential (LFP) DBS leads macro-stimulation (for chronic stimulus) recordings prior to and throughout STN-DBS), and characterize input and output (I/p and O/p) relationship of BG nuclei and cortex, with and without deep brain stimulations. We will extend our preliminary work, wherein we successfully employed PCA method to characterize spike train dynamics in the STN. [17] We propose to use MIMO point process models (Figure 9, see Appendix) of Dr. Sharma`s laboratory,[2], [9], [10] (John`s Hopkins University, and Dr. John Gale Cleveland Clinic)). We will also use STN data recorded by Dr Emad Eskandar MD, Surgical Specialist, and Neurological Surgeon in Boston, Massachusetts General Hospital, MA, Cambridge, USA.
Another important technique is the autoregressive modeling (AR). The AR modeling is widely used in several indeed many domains. [47] Cassidy, et al [56] employed a variation of the method proposed, but chose a different route for analysis. They used dynamic multivariate auto regressive (MAR) models to investigate the non-stationary coherence among LFP signals in the STN and GPi, and with electroencephalography (EEG) signals.
We have taken that analysis in a different direction by producing state space system representations of the AR models and analyzing the transition matrix properties in the state evolution equation. As seen above, the maximum singular value feature of the AR-based state space models depict the most significant disparity between unaffected and affected GPi regions, indicating that the max(σ(A)) may have broader underlying meaning in the context of LFP analysis. In system’s theory, max(σ(A)) indicates the level of temporal dependencies in the data.
Could this mean that the response of the GPi on LFP signals is muted in regions corresponding to muscle groups afflicted with dystonia or that the neural circuit loses memory in afflicted regions? To better decipher the system’s response characteristics, it is necessary to control the input signal by way of modulating LFP activity within the GPi. However, due to limitations on intra-surgical experimentation methods, we were unable to generate the system input stimulus required to build more complicated models.
Appendix
Author Contrubution
This study designed and developed by the first author and the inputs - interpretation and analysis and also computational work done by the rest of the authors.
Source of Funding
DST-CSRI Funded by the Ministry of Science & Technology (MST), Government of India, New Delhi.
Conflict of interest
None
References
- Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932-42. [Google Scholar]
- Connolly AT, Muralidharan A, Hendrix C, Johnson L, Gupta R, Stanslaski S. Local Field potential recording in a non-primate model of Parkinson`s disease using the Activa® PC+S neurostimulator. J Neural Eng. 2019;12(066012):1-25. [Google Scholar]
- Morishita T, Inoue T. Need for multiple biomarkers to adjust parameters of closed-loop deep brain stimulation for Parkinson's disease. Neural Regen Res. 2017;12(5). [Google Scholar]
- Telkes I, Viswanathan A, Jimmenez-Shahed J, Abosch A, Ozturk M, Gupte A. Local field potentials of subthalamic nucleus contain electrophysiological footprints of motor subtypes of Parkinson’s disease. Proce National Acad Sci. 2018;115:E8567-76. [Google Scholar]
- Zhao D, Sun Q, Cheng S, He M, Chen X, Hou X. Extraction of Parkinson`s Disease-Related Features from Local Field Potentials for Adaptive Deep brain Stimulation. Neurophysiol. 2018;50(1):57-60. [Google Scholar]
- Parastarfeizabadi M, Kouzani AZ. Advances in closed-loop deep brain stimulation devices. J Neuro Eng Rehabil. 2017;14(1):1-20. [Google Scholar]
- Wang DD, Hemptinne Cd, Miocinovic S, Qasim SE, Miller AM, Ostrem JL. Subthalamic local field potentials in Parkinson's disease and isolated dystonia: An evaluation of potential biomarkers. Neurobiol Dis. 2016;89:213-22. [Google Scholar]
- Thompson JA, Lanctin D, Ince NF, Abosch A. Clinical Implications of Local Field Potentials for Understanding and Treating Movement Disorders. Stereotact Funct Neurosurg. 2014;92(4):251-63. [Google Scholar]
- Huberdeau D, Montgomery ;HWHHE, Sridevi ;, Sarma V. Analysis of Local Field Potential Signals: A Systems Approach, 33rd Annual International Conference of the IEEE EMBS. . 2011. [Google Scholar]
- Kane A, Hutchison WD, Hodaie M, Lozano AM, Dostrovsky JO. Enhanced synchronization of thalamic theta band local field potentials in patients with essential tremor. Exp Neurol. 2009;217(1):171-6. [Google Scholar]
- Sarma SV, Eden UT, Cheng ML, Williams ZM, Hu R, Eskandar E. Using Point Process Models to Compare Neural Spiking Activity in the Subthalamic Nucleus of Parkinson's Patients and a Healthy Primate. IEEE Trans Biomed Eng. 2010;57(6):1297-305. [Google Scholar]
- Saxena S, Schieber MH, Thakor NV, Sarma SV. Aggregate Input-Output Models of Neuronal Populations. IEEE Trans Biomed Eng. 2012;59(7):2030-9. [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18(4):357-63. [Google Scholar]
- Telkes I, Jimenez-Shahed J, Viswanathan A, Abosch A, Ince NF. Prediction of STN-DBS Electrode Implantation Track in Parkinson's Disease by Using Local Field Potentials. Front Neurosci. 2016;10(10):198-9. [Google Scholar]
- Meloni G, Sen A, Abosch A, Ince NF. Spatial distribution of nonlinear interactions in subthalamic nucleus local field potentials in Parkinson’s disease. 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2015. [Google Scholar]
- Thompson JA, Lanctin D, Ince NF, Abosch A. Clinical implications of local field potentials for understanding and treating movement disorders. Stereotact Funct Neurosurg. 2014;92:251-63. [Google Scholar]
- Abosch A, Timmermann L, Bartley S, Rietkerk HG, Whiting D, Connolly PJ. An International Survey of Deep Brain Stimulation Procedural Steps. Stereotact Funct Neurosurg . 2013;91(1):1-11. [Google Scholar]
- Abosch A, Hutchison WD, Saint-Cyr JA, Dostrovsky JO, Lozano AM. Movement-related neurons of the subthalamic nucleus in patients with Parkinson disease. J Neurosurg. 2002;97(5):1167-72. [Google Scholar]
- Raju VR, Mridula RK, Borgohain R. Effect of Microelectrode Recording in Accurate Targeting STN with High Frequency DBS in Parkinson Disease. IETE J Res. 2019. [Google Scholar]
- Parkinson J. An Essay on Shaking Palsy. . 1817. [Google Scholar]
- Benabid AL. Lasker~DeBakey Clinical Medical Research Award. Nat Med. 2014;20(10):xviii-xx. [Google Scholar]
- Shaikh AG, Mewes K, Jinnah HA, DeLong MR, Gross RE, Triche S. Globus pallidus deep brain stimulation for adult-onset axial dystonia. Parkinsonism Related Disord. 2014;20(11):1279-82. [Google Scholar]
- Raju BVR, Anuradha B, Sreenivas. Effectiveness of Lead Point with Microrecording for Determining STN-DBS in Parkinson disease using HM-Models. Int Res J Eng Technol (IRJET). 2019;6:1-9. [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74(3):449-57. [Google Scholar]
- Rossi L, Foffani G, Marceglia S, Bracchi F, Barbieri S, Priori A. An electronic device for artefact suppression in human local field potential recordings during deep brain stimulation. J Neural Eng. 2007;4(2):96-106. [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson's disease?. Ann N York Acad Sci. 2012;1265:9-24. [Google Scholar]
- AP, GF, AP, Tamma F, AMB, MP. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol. 2004;189(2):369-79. [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci. 2013;14(11):770-85. [Google Scholar]
- Schwartz AB, Cui XT, Weber D, Moran DW. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron. 2006;52(1):205-20. [Google Scholar]
- Deeb W, Giordano JJ, Rossi PJ, Mogilner AY, Gunduz A, Hudy JW. Proceedings of the fourth annual deep brain stimulation Think Tank, A review of emerging issues and technologies. Frontiers in integrative neuroscience. 2016;1:10-38. [Google Scholar]
- Hosain MK, Kouzani A, Tye S. Closed loop deep brain stimulation: an evolving technology. Australas Physical Eng Sci Med. 2014;37(4):619-34. [Google Scholar]
- Sarbaz Y, Pourakbari H. A review of presented mathematical models in Parkinson’s disease: black- and gray-box models. Med Biol Eng Computing. 2016;54(6):855-68. [Google Scholar]
- Faraji AH, Richardson RM. Deep Brain Stimulation in Early Parkinson Disease May Slow Rest Tremor Progression. Neurosurg. 2019;84(1):E13-4. [Google Scholar]
- Saara M, Rissanen M, Kankaanpää MP, Tarvainen V, Novak P, Novak K. Karjalainen Analysis of EMG and Acceleration Signals for Quantifying the Effects of Deep Brain Stimulation in Parkinson’s Disease. IEEE Trans Biomed Eng. 2011;58(9). [Google Scholar]
- Levin J, Krafczyk S, Valkovič P, Eggert T, Claassen J, Bötzel K. Objective measurement of muscle rigidity in parkinsonian patients treated with subthalamic stimulation. Mov Disord. 2009;24(1):57-63. [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Dis-ord. 2003;18:357-63. [Google Scholar]
- Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp Neurol. 2005;194(1):212-20. [Google Scholar]
- Ray N, Jenkinson N, Wang S, Holland P, Brittain J, Joint C. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson's disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp Neurol. 2008;213(1):108-13. [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson's disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380-7. [Google Scholar]
- Weinberger M, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic re-sponse in Parkinson’s disease. J Neurophysiol. 2006;96:3248-56. [Google Scholar]
- Pogosyan A, Yoshida F, Chen C, Martinez-Torres I, Foltynie T, Limousin P. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization. Neurosci. 2010;171(1):245-57. [Google Scholar]
- Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurg. 2012;71:804-14. [Google Scholar]
- Foffani G, Ardolino G, Egidi M, Caputo E, Bossi B, Priori A. Subthalamic oscillatory activities at beta or higher frequency do not change after high-frequency DBS in Parkinson's disease. Brain Res Bull. 2006;69(2):123-30. [Google Scholar]
- Grundmann K. Primary Torsion Dystonia. Arch Neurol. 2005;62:682-5. [Google Scholar]
- Jancovic J. A textbook of movement disorders. . 2007. [Google Scholar]
- Jancovic J. Parkinson's Disease and Movement Disorders. Lancet Neurol. 2008;7(1):8-11. [Google Scholar]
- Masimore B, Kakalios J, Redish A. Measuring fundamental frequencies in local field potentials. J Neurosci Methods. 2004;138(1-2):97-105. [Google Scholar]
- Martens H, Toader E, Decré M, Anderson D, Vetter R, Kipke D. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol. 2011;122(3):558-66. [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber S, Israel Z. Closed-Loop Deep Brain Stimulation Is Superior in Ameliorating Parkinsonism. Neuron. 2011;72(2):370-84. [Google Scholar]
- Raju VR. A Study of Multi-Channel EMG in Writer`s Cramp”, a PhD Thesis submitted to the Nizam`s Inst of Medical Sciences. . 2009. [Google Scholar]
- Cordivari C, Lees AJ, Misra VP, Brown P. EMG-EMG coherence in writer's cramp. Mov Disord. 2002;17(5):1011-6. [Google Scholar]
- Grosse P, Edwards M, Tijssen M, Schrag A, Lees AJ, Bhatia K. Patterns of EMG-EMG coherence in limb dystonia. Mov Dis. 2004;19(7):758-65. [Google Scholar]
- Cotzias GC. Lasker Awards:1969 Albert Lasker Clinical Medical Research Award. L-Dopa for treating Parkinson’s disease For the demonstration of the effectiveness of L-DOPA in the treatment of Parkinson’s disease. . 1969. [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiology-Heart Circ Physiol. 2000;278(6):H2039-49. [Google Scholar]
- Hans S, Kim D, Kim H, Park JW, Youn I. Electrical stimulation inhibits cytosine arabinoside-induce neuronal death by preventing apoptosis in dorsal root ganglion neurons. Neuroreport. 2016;27(6):1217-41. [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Lazzaro VD. Movement‐related changes in synchronization in the human basal ganglia. Brain. 2002;125(6):1235-46. [Google Scholar]
- Abstract
- Introduction
- Aims and Objectives
- Deep Brain Stimulation Methodology
- Methods
- Local Field Potentials and their functional characterizations and inferences in Parkinson`s Disease
- Higher-Frequency Fluctuations
- Dyskinesia
- Resting-tremor
- Postural Instability and gait difficulty
- Essential-tremor and local fields
- Dystonic movement and field potentials
- Local field potential recordings
- Adaptive Deep Brain Stimulators
- Coherence
- Coherence model estimation
- Standard deviation
- Root mean square
- Entropy
- Discussion, Conclusions and future directions
- Appendix
- Author Contrubution
- Source of Funding
- Conflict of interest
- References
How to Cite This Article
Vancouver
Raju VR, Konda S, Balmuri KR, Balabhadra A, Raju B. MER based analysis of local field potentials with deep brain stimulation subthalamic nucleus in Parkinson/'s disease using coherence and entropy techniques [Internet]. IP Indian J Neurosci. 2020 [cited 2025 Oct 16];6(3):202-219. Available from: https://doi.org/10.18231/j.ijn.2020.041
APA
Raju, V. R., Konda, S., Balmuri, K. R., Balabhadra, A., Raju, B. (2020). MER based analysis of local field potentials with deep brain stimulation subthalamic nucleus in Parkinson/'s disease using coherence and entropy techniques. IP Indian J Neurosci, 6(3), 202-219. https://doi.org/10.18231/j.ijn.2020.041
MLA
Raju, Venkateshwarla Rama, Konda, Srinivas, Balmuri, Kavitha Rani, Balabhadra, Anvesh, Raju, BSV. "MER based analysis of local field potentials with deep brain stimulation subthalamic nucleus in Parkinson/'s disease using coherence and entropy techniques." IP Indian J Neurosci, vol. 6, no. 3, 2020, pp. 202-219. https://doi.org/10.18231/j.ijn.2020.041
Chicago
Raju, V. R., Konda, S., Balmuri, K. R., Balabhadra, A., Raju, B.. "MER based analysis of local field potentials with deep brain stimulation subthalamic nucleus in Parkinson/'s disease using coherence and entropy techniques." IP Indian J Neurosci 6, no. 3 (2020): 202-219. https://doi.org/10.18231/j.ijn.2020.041
