Introduction
Pyriformis syndrome (PS) was first described in 1934 by Freiberg and Vinke1 who attributed mechanical compression of the sciatic nerve (SN) by the pyriformis muscle (PS) as a cause of mechanical low backache (LBA) and sciatica. In the subsequent decades, reams of literature have been written about this entity with opinions ranging from outright scepticism2 to those suggesting higher prevalence and underdiagnosis of PS.3 Various treatment modalities for PS have also been described ranging from mechanical manipulation, injection of steroid and anaesthetic agents and surgical decompression.4 We describe the short term and intermediate efficacy of direct intra-muscular injection of botulinum toxin (BTX) in PS along with the safety profile.
Materials and methods
All patients referred for Neurosurgery, Neurology and Rheumatology OPD for chronic low backache and sciatica were carefully screened clinically and with MRI to exclude other causes such as intervertebral disc herniation, sacroiliitis, facetal arthropathy and lumbar canal stenosis. A diagnosis of pyriformis syndrome was made as a diagnosis of exclusion only after careful clinical assessment. Initial trial of conservative treatment with analgesics and physiotherapy was prescribed. Patients were selected for botox infiltration if adequate symptom relief was not noted after conservative management.
Technique of Pyriformis Botox infiltration
All procedures were done on an out-patient basis. Patients were placed prone on the CT table with a pillow placed under the lower pelvis for elevation of the pelvis and the hip joints. Following topogram of the pelvis, limited non-contrast CT scan (NCCT) of the pelvis was performed. The pyriformis muscle (on the symptomatic side) was localised and the course of the sciatic nerve was traced to look for variants and intra-muscular course. Subsequently a total of 03 spinal needles (22 G) were placed in the pyriformis muscle belly, at a distance of 1 to 1.5 cm apart. Limited check NCCT was done to confirm good intra-muscular position of the needles and safe distance of the needle tips from the sciatic nerves (Fig 1). Subsequently 50U of Botox was injected through each of the needles into the muscle belly (total dose of 150 U). Aspiration was done prior to injection to prevent inadvertent intra-vascular injection. Patient was asked to continue lying prone for 05 minutes after the injection. Patient was asked to perform flexion movement at the hip and foot immediately and 5 minutes after the injection. Patients were subsequently gently ambulated and after ascertaining the absence of neurological deficits, were allowed to return home. Clinical review was obtained after 03 days, 03 months and 06 months. Patients were asked to report for clinical review beyond 6 months in the event of symptoms recurrence.
Data analysis
Symptom assessment was done using the Visual Analogue Scale (VAS)5 for pain. The assessment was done before the procedure as well as on post procedure reviews at 3 days, 3 months and 6 months. The VAS was designed as a straight horizontal line measuring 100 mm. The ends of the line were defined to imply absence of pain (left side) with progressively increasing pain severity towards the right with the right end of the line implying worst possible pain. The treated patients were made to demarcate a point on the line which according to the individuals represented the intensity of pain. The pain score was calculated by measuring the distance in millimetres from the left edge of the line to the point marked by the patient. 5
The initial and subsequent follow up pain score results were used to grade the efficacy of treatment as a percentage of the initial (pre-treatment) pain score:
Failure of treatment was described when patients did not have at least one grade of improvement of symptoms at 3 or 6 months from treatment.
Results
A total of 26 procedures were performed on 25 patients (15 male, 10 female) with one patient receiving a second procedure 15 months after the initial treatment. The mean age of the patients population was 48.6 years (Range 23 to 72 years). No procedure related complications were noted and all patients were successfully ambulated immediately after the procedure.
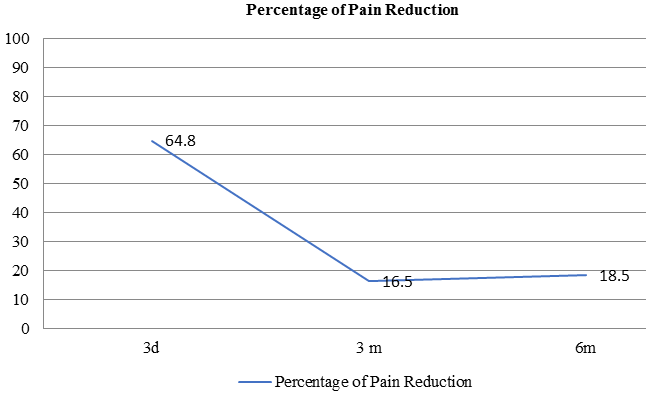
The mean percentage pain relief at 3 days, 3 months and 6 months was as detailed inTable 1.
Figure 1
Axial CT image showing position of needles within the right pyriformis muscle (thick white arrow). The right sciatic nerve is marked with the thin white arrow.

Table 1
| 3 Days | 3 months | 6 months | |
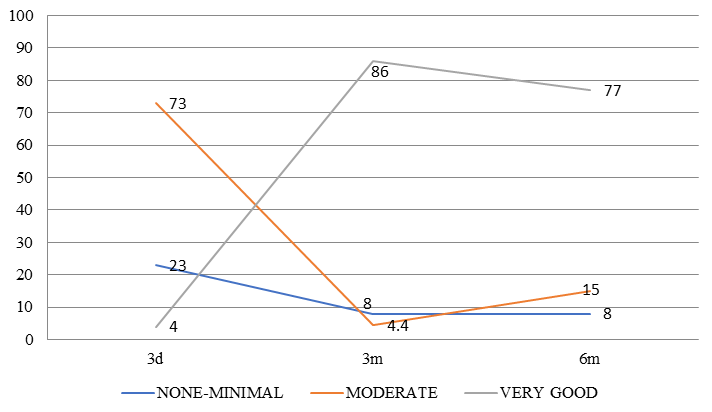
| None / Minimal(<20%) | 6/26 (23.1%) | 2/26 (7.7%) | 2/26 (7.7%) |
| Moderate (20 to 80%) | 19/26 (73.1%) | 1/26 (3.8%) | 4/26 (15.4%) |
| Very Good (>80%) | 1/26 (3.8%) | 23/26 (88.5%) | 20/26 (76.8%) |
The degree of pain relief experienced by the patients (expressed as a percentage) over the period of follow up is mapped in Figure 2, Figure 3 .
Failure of treatment was noted in 2 cases as detailed in Table 1. Both patients had mild to no relief at 3 months and 6 months after treatment. Both patients had lumbar disc protrusions on MRI with symptoms which were equivocal for pyriformis syndrome and lumbar radiculopathy. Pyriformis infiltration was attempted after failure of initial conservative management and individuals unwilling for surgical treatment despite severe clinical symptoms.
Discussion
The pyriformis is a triangular sheet like muscle which runs from the anterolateral surface of the sacrum, through the greater sciatic notch and inserts on the greater tuberosity of the humerus. Its primary actions are external rotation of the extended hip and abduction of the flexed hip.6 The PM is closely related to the SN along its course with as many different anatomic variations being described.5 PS as a cause of sciatica was first described by Freiberg and Vinke in 1934 who attributed a mechanical compression of the nerve by the overlying muscle as a direct cause of pain.1 Over the subsequent decades, a number of mechanisms for pyrifomis syndrome were postulated. The more common of these are mechanical compression of the SN by the PM, enlargement and inflammation of the pyriformis as a result of abnormal gait and or repeated prolonged sitting on hard and uneven surfaces, therefore aptly termed “wallet neuritis”.4 The diagnosis of PS is often a clinical conundrum as the symptoms often mimic other causes of sciatica such as lumbosacral discogenic pain. Various other musculoskeletal pathologies such as sacroiliitis also mimic the symptoms of PS. In view of the same, the diagnosis of PS has been viewed with scepticism and suggestions that its prevalence is more than described and that the condition is underdiagnosed. The diagnosis of PS is predominantly clinical with imaging performed to rule out other causes. A number of clinical tests have been described for the diagnosis of PS in addition to the occasionally elicitable tender and inflamed PM.7 In our series, all patients underwent careful clinical examination and an MRI of the lumbosacral spine and the SI joints to exclude other pathologies. Electromyography (EMG) has displayed prolonged F response and H reflex in the SN territory to be specific to PS, however many documented patients have normal EMG studies.8 Electrophysiological tests were not mandatory prior to treatment in our patient cohort.
A variety or treatment modalities for PS have been tried over the years as summarized in Table 2. The main target of treatment is to lessen the inflammation and spasm in the muscle. Most series have described an initial trial of anti-inflammatory (non-steroidal) drugs initially followed by other forms of treatment when the former does not succeed.
Table 2
Different modalities of management in PS.
Botulinum toxins (BTX) are neurotoxins produced by multiple species of the spore forming bacterial genus Clostridium, including the eponymous Clostridium botulinum.15 These toxins have been traditionally sub-divided into 6 different types (A to F) although only types A and B are commercially available for pharmacological use. The BTX is a protein comprising a heavy and a light chain.16 The heavy chain serves to internalize the BTX molecule into the neuron in an intra-vesicular form and subsequently causes release of the light chain into the neuronal cytoplasm. The light chain then attaches to specific receptors causing release of acetylcholine from vesicle.17 The resultant clinical manifestations are muscle weakness and eventually complete paralysis. After the commercial production of BTX, it found multifold use in varying fields like cosmesis, neurology, gynecology, pain syndromes and opthalmological disorders.18 In the field of neuroscience, it has been used in the treatment of various conditions associated with spasticity.19
The first study specifically describing its use in PMS was published by Fanucci et al who reported both a safe and efficacious modality of treatment.20 A number of studies have subsequently validated these results21, 22, 23 The largest such series by in 122 cases showed good / very good pain relief as per the VAS in 77% cases. These results corroborate with our results in which more than 80% patients had very good pain relief at 3 months and more than 75% patients at 6 months after the procedure.
Positioning of needles within the muscles has been described by manual palpation, EMG guidance and image guidance (CT / USG). We found CT guidance to be extremely useful for confirming optimal needle positioning as well as confirming safety profile in view of proximity to the sciatic nerve. While no intra-muscular course of the nerve were noted in our series, we postulate that CT is the best modality for tracing the entire SN course in relation to the muscle and avoid inadvertent nerve injury during the procedure.
The ideal location for intramuscular injections debatable, however most practitioners advocate needle placement within the belly of the muscle. Some advocate a relatively medial injection with direct palpation of the muscle, while others prefer a lateral injection which provides a better safety profile in view of the proximity to the SN.4 We found CT guidance to be an extremely effectively method of localizing the entire muscle and the nerve. 03 needles could be placed in the muscle belly from medial to lateral to provide uniform infiltration. No procedure related complications were noted in any or our patients which was consistent with the available literature describing a high safety profile.
The main limitation of our study was availability of a relatively short follow up period (6 months). Studies with longer follow up periods are required to estimate the long term efficacy, incidence of symptom recurrence and need for retreatment.
Conclusion
CT guided Botox infiltration into the pyriformis is an effective modality for short term and intermediate pain relief in pyriformis syndrome. Proper clinico-radiological assessment is imperative to exclude other causes of pain. Three needle technique with infiltration across the length of the muscle might be more effective in intermediate and long term symptom alleviation.