- Visibility 114 Views
- Downloads 51 Downloads
- Permissions
- DOI 10.18231/j.ijn.2022.026
-
CrossMark
- Citation
Analysis of real-time multi channel microelectrode recordings of rats: A study with computational simulation
- Author Details:
-
V Rama Raju *
Abstract
This study discuss the analysis of a real-time multi channel microelectrode recordings of rats with computational simulation and mathematical statistical modeling’s. If the impedance-of every site of neuro-sensor, i.e., electrode is fairly at a low level as well as the gap between sensor-sites is applicably very very minute, a spike-generated and also computed by a neuron is asynchronously/concurrently gathered at multi neuro-sensor/electrode locations in conjunction with distinct stimulus-intensities, i.e., amplitudes (stremgth of the signals).
Introduction
Split of signal-spikes computer simulated-generated adjoining-neurons is a key problem in extra cellular micro electrode signal-recording from cell-dense-areas of the animal-brain (for instance, hippo-cmapul pyra midal cells). Even though we have different types of software, hardware, firmware windowing-spectral minimization methods, the number of neural cells distinguishable as of single unit single channel signal recordings is small, be in the region of two.[1] Normal standard-procedures can not be distinguished amongst analogous- stimulus-amplitudes/spikes initiating as of or on/ or after distinct-neurons intermediate as of the stimulating-sensor and acquiring micro electrode. [2] However, while latest multi channel spikes/data is to be grouped for clustering purposes, such technique needs the effect of preceding, ie.,eralier clustering-of-spike-data gathered under identical-conditions (the identical-electrode, multi neuronal locations, etc.). Such type of necessity enforces and necessitates the constraints or restrictions on the applicability-of the technique to a range-of multi channel spikes-data.
Therefore, we suggest at this juncture a significant enhancement of the stereo trode technique applying through the co-variance, instead of simply the peak-amplitude-ratio, amongst a pattern-tmplate/signature plus a definite spike-acquired by using a multi site micro electrode. [3]
Goal
To explore the multi unit multi site micro electrode signal acquisitions of rats hippocampus pyramidal cell layers as well as deep-brain stimulation in anesthetized non-primate-animals using artificial intelligence/machine-learning-techniques (both supervised and unsupervised), signal, as well as clustering-methods..
Methods
Standard traditional windowing-methods distinguish neuronal-spikes by means of referencing their highest-signal-amplitudes primely. Though, limitations of spike-stimulus-amplitudes in the middle of numerous-cells-neurons are complicated to ascertain, e.g., two neural-cells/neurons which are intermediate after the sensor-site might have virtually indistinguishable stimulus-amplitudes/signal-strengths or power of the signal. Mc Naugton,et.al. established the stereo trode technique wherein neuronal-spikes are divided by referring to the probabilistic joint-distribution of highest/peak-amplitudes values achieved by multi channel signal recordings.[2] The following [Figure 1] depicts is derived which is a combination of signal-decompositin/.

Idea
If a succulently strong stimulus is applied to whatsoever part of our or animal body then it gives rise to some excitation and this excitation is due to ion permeability of the membranes. i.e., fluctuations in the membranes. The electrical-action potentials of the aimed neuron is acquired extra cellularly in conjunction with a multi site micro electrode ([Figure 2]).
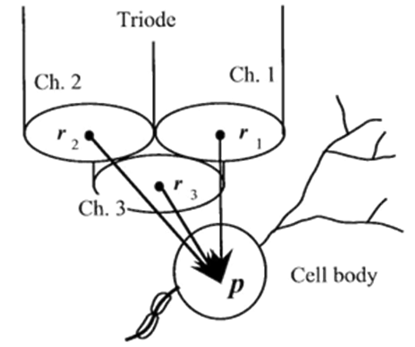
Let us consider a spike-generated at the length of time ”T” passing through the aimed neural-cell/ the-neuron positioned at a ‘point’ “p” is derived as
v_n (t) = h (t, p -r_ n) x w (t - T)........ (1)
By way of focussing on the mitigation/ effect of the spatial (temporal) damping factor, the equation (1) is converted in to the
v_ n(t) = h (p - r_ n). w (t – T) + ε_ n (t) ......(2)
By means of minimizing the energy of the error-function, is projected as and then the equation
v_ n (t) ≅ h (p - r_ n). w (t - T) ......(3)
is normalized so that its negative roughly equalizes the -Vepeak of signal-amplitude of evry neural-spikes.
H ̂(p)=(h ̂(p-r_1), (h ̂(p-r_2), (h ̂(p-r_3), … , (h ̂(p-r_N) (4)
[4], template-signal/wave forms ought be carefully-chosen, contemplating the range of examined spike of the signal-waveforms [5].
Based-on the unique-template, elastic-template
is derived as w ^γ = ψ (t / γ)...........(5)
where indicates the duration ratio of to i.e., the resistance of is described by the optimum value of is determined so that each spike detected has a maximum-correlation with optimized
Identifying the spikes
Thecorrelation-technique has been extensively applied for identifying neural/neuronal-spikes.[4] In this, the acquired-signals-having high-correlation-coefficients in conjunction through a pattern-matching-template are identified as signal-amplitudes-spikes. [4], [5], [6], [7]
The following [Figure 3] shows one of the correlation-coefficients-whenexceeds, a amplitude of the signal-spike is believed to have been identified.
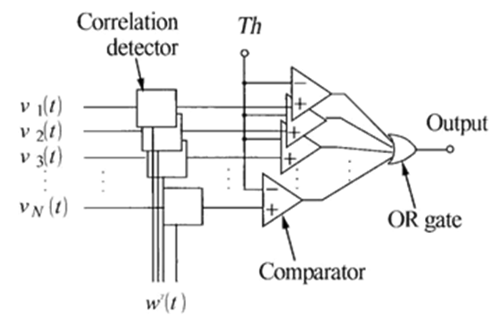
Correlation— coefficient computation
To enhance detection of the signal spikes of waveforms, the weight -v e c t o r functor can be used for computation of the c o r r e l a t I o n-c o e f f i c i e n t s. Consequently, by employing the weight functors, the processed—signal at the n - th electrode -point wn(t) comprises analogous overlap and correlating-spikes
vn= ∑j=-∞+∞vj, n(t)....................(6)
Where in, vj, n t yielding the j-th interpreted spike formed through some neuronal cells inside the region of the n - th electrode- point at Ti time Ti. Consequently, conceited that the weight- functor Wγ(t) attenuate the neural-signal-spikes happening beforehand plus following the neural-spike witnessed at , by means of neuronal-spike duration-ratio ‘’. Here, we applied a smoothed-square-waveform of the elastic-template for weighting
wγ(t) = ∫-δ8+δ8wγ(t+τ)2dτ∫-δ8+δ8wγ(τ)2dτ..............(7)
i.e.,
Wγ(t) = ∫-δ8+δ8wγt2+τ2+2tτdτ∫-δ8+δ8wγ(τ)2dτ........(8)
δ-showing the length of the period of the neural-spike-train that is 1 ± 3 milli-seconds. (± 1 mil Second to ± 3 mil Seconds). Through this weighting- vector allowing us to separate the neural/neuronal-spike observed at Tk as given in the following expression (9)
vk,n(t) ≅ vn(t) . Wγ(t-Tk).........(9)
Thus, can change the pattern/signature -signal pattern-template as
wγ(t) ≅ wγ(t) . Wγ(t - Tk)......(10)
We can now thus can generate/compute the correlation coefficient concerning vk,n(t) as well as the pattern-template (i.e.,the variable-template) as
Rk,nγ(t) = ∫-δ+δvk,nt+τ.wγ(τ)dτ∫-δ+δ vk,n(t+τ)2dT.∫-δ+δwγ(τ)2dτ .........(11)
By changing the expression (11) into the expression (12) through the application of expression (9), as proved in the equation shown below
Rk,nγ ≅∫-δ+δvnt+τ.wγt+τ-Tk.wγt. Wγτdτ∫-δ+δvk,n(t+τ)2.Wγ(t+τ-Tk)2dτ. ∫-δ+δwγ(t)2. Wγ(t)2dτ Rk,nγk ≅ ∫-δ+δvnTk+t.wγkT.wγτ. Wγk(τ)2dτ∫-δ+δvn(Tk+t)2.Wγk (τ)2dT. ∫-δ+δwγk(τ)2. Wγk(τ)2dτ Rnγ = ∫-δ+δvnt+τ.wγτ. Wγ(τ)2dτ∫-δ+δvn(t+τ)2.Wγ (τ)2dT. ∫-δ+δwγ(τ)2. Wγ(τ)2dτRk,nγk(Tk) ≅ Rnγk(Tk).............. (15)
vn(k)(t) = ∑j≥k+∞vj,n(t) = vn(t)- ∑j=-∞k-1vj,n(t)
≅vn(t) – ∑j≥k+∞h^pj-rn . wγj(t- Tj,n)..........(16)
By connecting the vn(k)(t) as vn(t) in equation (14), the k-th neural-spike is characterized or distinguished from vn(k)(t) unaltered through positive (+Ve) deflections or refractions of previous train neural-spikes.
Damping factorial generation
Through the application of estimating functor
En( Tkn) = 12δ∫-δ+δε((Tkn+τ)2dτ
= 12δ∫-δ+δεvk,nTk,n+τ-hPk-rn.wγjτ2dτ .......(17)
Then the equation yield
dEn(Tk,n) dh(Pk-rn) = - 12δ∫-δ+δ2vk,nTk,n+T-hPk-rn.wγj(τ) .wγkTdτ=0 ..........(18)
vk,nTk,n+T-hPk-rn.wγjT= - 12δ∫-δ+δ2vk,nTk,n+τ . Wγkτ-hPk-rn.wγj(τ)Wγkτ .wγkτ.Wγkτ dτ≅0 .........(19)
And then the above expression, i.e., (19) can be computed and while computing it must be set to
h^Pk-rn∴h⏞(pk-rn) ≅∫-δ+δvk,nTk,n+τ .Wγkτ . Wγk(τ)2dτ ∫-δ+δwγk(τ)2. Wγk(τ)2dτ=h⏞ (pk-rn) ..... (20)
Hence, it is set to h^( pk- rn)
Now, if ∫-δ+δwγk(τ)2dτ=1, the expression (20) is identically equal to the covariance between vnTk,n+t and also wγkτ.
Spike train potentials
A pair-of-spikes is measured to happen in a gust-under the subsequent constraints: the inter-spike/interval (I S I) is I S I_ b_min, ISI b_max), the corr-coefficient of damping-vectors is (C.C.b_min,1) and attenuation ratio-of-spike amplitudes/signal-strengths ar (attnb_min,attnb_max) which is depicted in the following [Figure 4].
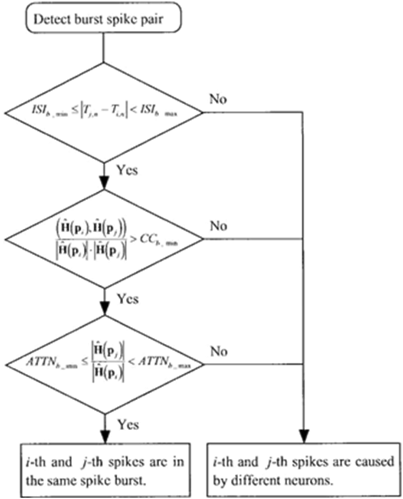
Conglomerate-clustering
The cluster is considered as a normal-distribution of N(H; μi, Σi):
N(H; μi, Σi) = 12π2Σie-12H-μiTΣi-1(H-μi) ......(21)
Pi,j ≅ (1/2)i
Pi,j ≅ (1/2)∫Iμi-H|>| μj-H|NH;μi,∑i,jdH+∫Iμi-H|>| μj-H|NH;μi,∑i,jdH
∫∞-12μi-μjτ∑i,j-112μi-μjN(h;0,1)dh≅∫-∞-12μi-μjτμi-μj-1∑i,j-1μi-μjN(h;0,1)dh
............(22)
μ i -μ jT12(Σ I+ Σ J) - 1≤4
Acquisition of spikes-data
A triode/ or a 7 – core - electrode was employed as a multi-site-electrode which consists of a pack of 3 fine stainless-steel-wires ([Figure 2] ; 50 μ-diameter, ~50k𝝮 -impedance at 1kilo-Hz). The cross section of each wire was employed as the detection area-of-neuronal activity. The 7-core-electrode was a quartz-coated-electrode through 7-core-platinum/tungsten ( 9010) cores ([Figure 5],1±2Meg𝝮 -impedance at a 1kilo Hz).
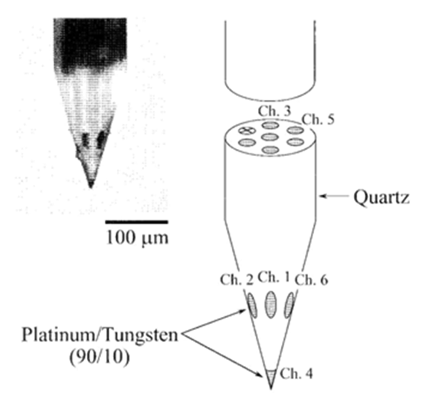
Results and Discussion
This study discussed the equality of a clusters/group with a hippo-campus neuronal or neural-cells. But, the rationality of this conception has not yet resolute. We skilled additional supplementary explorations in which the targeted neuron was distinguished by the collision test. The damping-vectors were resolute by our grouping technique. Subsequently the damping-vectors of the perceived neuronals neuron/neural-cell distilled in a clustering-group that interconnected thriving to one of the cluster/groups in the multi-cluster/group distribution, representing the neuron-cluster communication.
Conclusion
The computational simulation and statistical modeling helps a lot in medical diagnosis especially for effective medical diagnostics in connection with the Brain disease and disorders like Parkinson`s disease, Alzheimer`s, cancer malignancy. So the developed simulation procedure for asynchronously classifying many neural-spikes and neuronal-spikes as of multi site multi-real time channelogrphs of the rats-brain. This simulated technique is more effective and more efficient in investigating multi neural connections in nearby linked neuronal networks.
Conflict of Interest
The researcher claims no conflict of interest.
Source of Funding
None.
References
- Park K. Clinical outcome prediction from analysis of microelectrode recordings using deep learning in subthalamic deep brain stimulation for Parkinson‘s disease. Plos One. 2021;16(1). [Google Scholar] [Crossref]
- Koirala N. Acquisition of MER Data of NEURON neurons with deep brain stimulation in Parkinson`s. Scientific Reports. 2020;10:1-13. [Google Scholar]
- Barbe M, Dembek T, Becker J, Raethjen J, Hartinger M, Meister I. Individualized current-shaping reduces DBS-induced dysarthria in patients with essential tremor. Neurology. 2014;82(7):614-9. [Google Scholar] [Crossref]
- Barbe MT. Multiple source current steering-a novel deep brain stimulation concept for customized programming in a Parkinson’s disease patient. Park Relat Disord. 2014;20(4):471-3. [Google Scholar] [Crossref]
- Berenstein C, Mens L, Mulder J, Vanpoucke F. Current steering and current focusing in cochlear implants: comparison of monopolar, tripolar, and virtual channel electrode configurations. Ear Hear. 2008;29(2):250-60. [Google Scholar] [Crossref]
- Blomstedt P. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. 2016;25(10):1350-6. [Google Scholar]
- Bonham BH, Litvak L. Current focusing and steering: Modeling, physiology, and psychophysics. Hear Res. 2008;242(1-2):141-53. [Google Scholar] [Crossref]
- Bour L, Lourens M, Verhagen R, Bie Rd, Munckhof Pvd, PS. Directional Recording of Subthalamic Spectral Power Densities in Parkinson's Disease and the Effect of Steering Deep Brain Stimulation. Brain Stimulati. 2015;8(4):730-41. [Google Scholar] [Crossref]
- Breiman L. Random Forests. Mach Learn. 2001;45:5-32. [Google Scholar] [Crossref]
- Buhlmann J, Hofmann L, Tass P, Hauptmann C. Modeling of a segmented electrode for desynchronizing deep brain stimulation. Front Neuroeng. 2011;4. [Google Scholar] [Crossref]
- Butson C, Cooper S, Henderson J, McIntyre C. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34(2):661-70. [Google Scholar]
How to Cite This Article
Vancouver
Raju VR. Analysis of real-time multi channel microelectrode recordings of rats: A study with computational simulation [Internet]. IP Indian J Neurosci. 2022 [cited 2025 Oct 09];8(2):125-129. Available from: https://doi.org/10.18231/j.ijn.2022.026
APA
Raju, V. R. (2022). Analysis of real-time multi channel microelectrode recordings of rats: A study with computational simulation. IP Indian J Neurosci, 8(2), 125-129. https://doi.org/10.18231/j.ijn.2022.026
MLA
Raju, V Rama. "Analysis of real-time multi channel microelectrode recordings of rats: A study with computational simulation." IP Indian J Neurosci, vol. 8, no. 2, 2022, pp. 125-129. https://doi.org/10.18231/j.ijn.2022.026
Chicago
Raju, V. R.. "Analysis of real-time multi channel microelectrode recordings of rats: A study with computational simulation." IP Indian J Neurosci 8, no. 2 (2022): 125-129. https://doi.org/10.18231/j.ijn.2022.026
