- Visibility 379 Views
- Downloads 46 Downloads
- Permissions
- DOI 10.18231/j.ijn.2023.040
-
CrossMark
- Citation
A comparative evaluation of microsurgical excision of olfactory groove meningioma through unilateral (pterional) vs Bilateral (Bifrontal-transbasal) approach
Abstract
Background: Surgical management of olfactory groove meningiomas poses significant challenges. Common microsurgical approaches often result in delayed exposure of neurovascular structures. In contrast, the pterional approach offers the advantage of early dissection of the posterior neurovascular complex.
Aim: Olfactory groove meningiomas constitute 4 to 13% of all meningiomas. Surgery is the primary treatment, but the recommended extent and types of approaches vary. We conducted a retrospective review of our olfactory groove meningioma series treated with microsurgery via standard unilateral or bilateral approaches.
Materials and Methods: Patient records from our department (RMLIMS, LUCKNOW) were reviewed, encompassing cases treated with unilateral or bilateral approaches. Thirty patients who underwent olfactory groove meningioma removal were included, and clinical data, radiological findings, surgical treatment, and clinical outcomes were retrospectively analyzed.
Results: A total of 30 craniotomies were performed, with 16 employing unilateral pterional approaches and 14 using bilateral transbasal approaches. Overall, gross total tumor resection was achieved in 27 cases. Postoperative complications included edema (in three patients) and hematoma (in three patients).
Conclusion: The unilateral (pterional) approach emerges as an excellent solution for olfactory groove meningioma treatment, offering early visualization of the posterior neurovascular complex. Additionally, it enables frontal sinus preservation and minimizes excessive brain retraction.
Introduction
Olfactory groove meningiomas (OGMs) constitute 4-13% of all intracranial meningiomas, originating from the dura of the anterior cranial fossa over the cribriform plate and frontosphenoidal suture.[1], [2], [3], [4], [5] Typically, OGMs manifest with hypo/anosmia, visual deterioration, mental changes, and headaches due to compression of the olfactory or optic nerve and frontal lobe. Seizures are also prevalent in these patients. [6], [7] Despite their slow growth, OGMs often remain clinically silent in their early stages, resulting in substantial tumor sizes at the time of diagnosis. [6], [7], [8], [9]
Distinguishing OGMs from other midline meningiomas in the anterior cranial fossa, such as those in the planum sphenoidale and tuberculum sellae, is not merely an anatomical exercise but holds clinical significance. Meningiomas in these locations are commonly diagnosed earlier due to visual impairment.
The classical bifrontal craniotomy proves inadequate for safe exposure of large OGMs, evident from the incidence of life-threatening complications related to brain retraction. [1], [4], [5] Alternative surgical routes, including the pterional and subfrontal approaches, [1], [10], [11], [12], [13], [14], [15] expose the posterolateral surface of the tumor from a lateral view. The fronto-basal-orbital approach [5], [16] accesses the tumor from underneath, exposing its dural attachment first.
In our retrospective analysis of 30 OGM cases, we assessed clinical presentation, tumor characteristics, surgical approaches, and follow-up results following microsurgical intervention through a unilateral (pterional/subfrontal) or bilateral (bifrontal) craniotomy.
Materials and Methods
We scrutinized the records of patients treated for olfactory groove meningioma at our department (RMLIMS, Lucknow) through unilateral and bilateral approaches. A total of 30 patients who underwent OGM removal were reviewed, and their clinical data, radiological findings, and surgical outcomes were retrospectively analyzed.
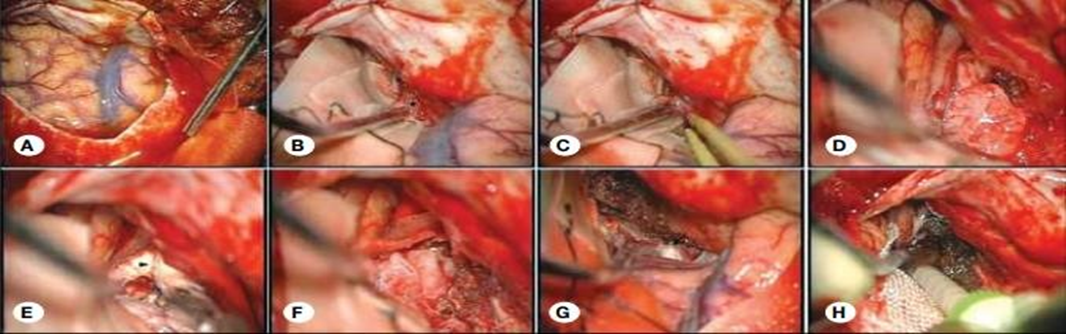
We identified 30 patients with olfactory groove meningioma (18 females and 12 males), aged between 33 and 54 years, with a mean age of 41.4 years in the unilateral group and 40.4 years in the bilateral group. The preoperative median Karnofsky Performance Score (KPS) was 100 (range, 60-100). Headache was the most frequent complaint (40%), followed by visual impairment (26.6%), anosmia/hyposmia (16.6%), and mental changes (6.6%). In 2 patients (6.6%), OGMs were associated with seizures ([Table 1]). Tumor diagnosis was based on magnetic resonance imaging (MRI) and/or computed tomography (CT) with a contrast agent, with two patients undergoing cerebral digital subtraction angiography (DSA) for embolization purposes. A total of 16 surgical procedures were conducted, utilizing both the pterional (n=16, 53.3%) and bifrontal (n=14, 46.6%) approaches. The pterional approach closely adhered to the technique originally described by Yaşargil. In this method, the frontal bone's lateral part, the anterior segment of the squamous part of the temporal bone, and the lateral aspect of the greater wing of the sphenoid bone were mobilized and excised. To facilitate improved access and manipulation of the anterior fossa from a broader perspective, the frontal part of the bone flap was extended medially and inferiorly. [Figure 1] illustrates the intraoperative steps following dural opening for this approach.
|
No. of Cases |
Female/ Male |
Anosmia |
Headache |
Decreased visual activity |
Hemiparesis |
Personality changes |
Seizure |
|
30 |
18/12 |
5 |
12 |
8 |
1 |
2 |
2 |
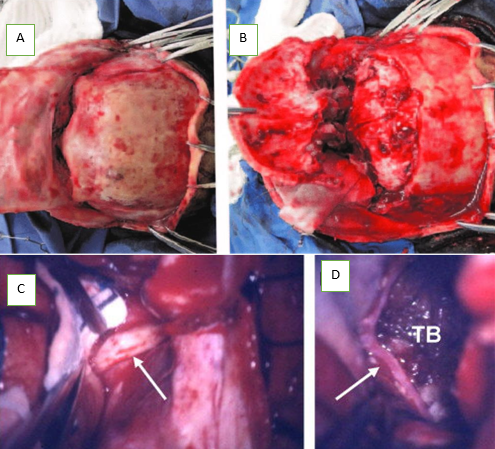
The bifrontal approach, closely mirrors the technique delineated by El Gindi. After a bicoronal scalp incision and periosteum dissection, a bifrontal bone flap was lowered to the orbital rim. The frontal sinus was opened, and its posterior wall and mucosa were excised, with subsequent closure of the frontonasal duct using muscle. Bilateral dural openings were performed, and tumor resection predominantly occurred from one side, with careful retraction of the non-dominant frontal lobe. The dominant frontal lobe's retraction was minimized as much as possible. In summary, a bifrontal craniotomy was executed after the release of supraorbital nerves. Subsequently, a bilateral orbitotomy was conducted, encompassing 2–2.5 cm of orbital roof crossing the midline just anterior to the crista galli. Post-procedure, the anterior skull base underwent reconstruction using a pedunculated flap of galea and abdominal fat to fill any residual dead space. ([Figure 2]).
Statistical analysis
Data analysis was conducted using the statistical software package SPSS 21.0 for Windows (SPSS Inc., Chicago, IL). Categorical variables were compared using Pearson’s chi-squared test and Fisher exact test. A significance level of P<0.05 was considered statistically significant.
Results
A total of 30 patients diagnosed with olfactory groove meningioma were included in the study, comprising 18 females and 12 males. The age distribution ranged from 33 to 54 years, with a mean age of 41.4 years in the unilateral group and 40.4 years in the bilateral group.
In our series, total removal was achieved in 27 patients, while partial removal occurred in three patients. Of the 30 OGMs, 16 (53.3%) were treated with the pterional -subfrontal approach, and 14 (46.6%) with the bifrontal transbasal approach. The pterional approach was significantly more frequent for small and medium-sized OGMs (p <0.05).
Overall, gross total resection (GTR) was achieved in 27 procedures (90%), with 10% classified as subtotal resection (STR). The pterional approach allowed a significantly greater percentage of GTR than the bifrontal approach (p<0.05). Simpson’s grade I-II resection was achieved in 27 procedures (90%), with the pterional approach showing a significantly higher percentage of Simpson I-II removal compared to the bifrontal approach.
Postoperative cerebral edema occurred in 12 patients, with a rate of 25% in patients with a unilateral approach and 57.14% in patients with a bilateral approach. The mean surgical length was 2 hours for the unilateral approach and 3 hours for the bilateral approach.
Surgical approach and complications
Postoperative complications were observed in 7 out of 30 operations (23.3%). Complications were seen in 2 (12.5%) and 5 (35.7%) patients operated via the pterional and unifrontal approach, respectively. Edema and hematoma with contusion were the most frequent complications. Overall, reoperation for complications was required in 1 case (3.3%).
Contusions observed in 3 patients were treated with medical intervention. Cerebrospinal fluid (CSF) leak was observed in one patient operated via the frontal route and was successfully treated with lumbar drainage. One patient experienced intracranial hematoma, requiring surgical evacuation.([Table 2], [Table 3])
|
|
Unilateral |
Bilateral |
|
Degree of removal |
|
|
|
Total removal |
15 |
12 |
|
Partial removal |
1 |
2 |
|
Post-operative edema |
4 |
8 |
|
Length of stay |
5 |
7 |
|
Surgical length |
2 |
3 |
|
Symptoms |
Preoperative signs and symptoms (number of cases) |
Postoperative resolution of signs and symptoms (number of cases still with symptoms) |
|
Olfactory deficits |
10 |
7 |
|
Headache |
8 |
2 |
|
Seizure |
5 |
0 |
|
Visual deficits |
4 |
2 |
|
Behavioral problems |
2 |
1 |
|
Hemiparesis |
1 |
0 |
Histological grading of tumors
According to the World Health Organization (WHO) classification, 28 tumors (93.3%) were Grade 1, and 2 (6.6%) were Grade 2. One of the Grade 2 meningiomas was associated with recurrence.([Table 4])
|
Resection level (Simpson grade) |
Unilateral (%) |
Bilateral(%) |
|
Grade 1 |
15(93.7) |
12( 85.7 ) |
|
Grade 2 |
|
|
|
Grade 3 |
1 |
2(0.14 ) |
|
Grade 4 |
(0.06 ) |
|
|
Grade 5 |
0 |
0 |
Treatment outcomes
In the median 60-month follow-up period, the overall tumor control rate was 96.8%. Recurrence and re-growth rates were 1.6% each, with a median follow-up to recurrence/re-growth of 31 months. Longer recurrence-free survival (RFS) was associated with GTR and WHO Grade 1 (p<0.05), while other factors showed no prognostic value on RFS, including age, sex, preoperative Karnofsky Performance Score, tumor size, type of approach, presence of ethmoidal invasion, optic canal involvement, vascular encasement, and hyperostosis.([Table 5])
|
Complications |
Unilateral |
Bilateral |
|
CSF leak |
0 |
1 |
|
Wound infection |
0 |
0 |
|
Postoperative edema |
1 |
2 |
|
Death |
0 |
0 |
|
Hydrocephalus |
0 |
0 |
|
Postoperative hemorrhage |
1 |
2 |
|
Visual loss |
0 |
0 |
|
Infarction |
0 |
0 |
|
Seizures |
0 |
0 |
Discussion
Francesco Durante successfully performed the first Olfactory Groove Meningioma (OGM) resection in 1885 through a left frontal craniotomy. [17] In 1938, Cushing and Eisenhardt outlined OGM resection principles in a series of 22 patients, using a unilateral subfrontal approach. [18], [19] Despite numerous publications on OGM treatment, the optimal surgical approach remains controversial. Pterional, frontal, bifrontal, and their variations are the most reported open transcranial procedures for OGMs. [1], [2], [3], [4], [5], [13], [15], [18], [20], [21], [22], [23], [24], [25], [26], [27], [28]
Nevertheless, the extensive dataset and long follow-up of OGM cases facilitate comparison of results with various surgical strategies. Key findings include: 1) higher mortality and severe complications with the bifrontal approach; 2) the pterional approach achieving a significantly greater percentage of Simpson I-II removal compared to bifrontal; 3) no retraction-related brain swelling in pterional cases, although postoperative CSF leak probability was higher with the bifrontal approach. Simpson grade I-II and WHO grade I were significant prognosticators for longer overall survival, with age and WHO grade being independent factors.
The pterional approach, introduced by Yaşargil, is widely used for vascular and neoplastic lesions in the anterior and middle cranial fossa. [29] Its advantages include early control of neurovascular structures, minimal damage to frontal lobes and olfactory nerves, and avoidance of frontal sinus transection, reducing the risk of CSF leak. [11], [13], [22], [30] However, it has a narrow working angle. The unifrontal approach, described by Olivecrona and Urban, spares the contralateral frontal lobe and superior sagittal sinus, avoiding bifrontal retraction and potential cognitive dysfunction. [5], [26] However, it has drawbacks like late exposure of distant neurovascular structures and the risk of CSF leak from frontal sinuses. [5], [22], [31]
The ongoing debate on surgical strategy focuses on tumor resection extent and safety. Our study showed a GTR rate of 90%, with no significant difference between pterional (93.75%) and bifrontal (85.7%) approaches. Optic canal involvement was associated with tumor diameter, while vascular encasement occurred in 8.2% of cases. Postoperative complications varied, with life-threatening complications more frequent in bifrontal cases. No mortality was observed in our series.[32], [33], [34], [35], [36], [37], [38], [39]
The most critical factor in preventing recurrence was the extent of initial resection, supported by a median follow-up of 60 months. Paranasal sinus infiltration increased recurrence risk, especially when involved bone was not removed.
Conclusion
In conclusion, our extensive series highlights the higher risk associated with the bifrontal approach and identifies age and WHO grade as independent factors affecting overall survival in OGM patients. While Simpson grade I-II's prognostic power may be influenced by other variables, it should continue to be the standard for OGM care.
Source of Funding
None.
Conflict of Interest
None.
References
- Bassiouni H, Asgari S, Stolke D. Olfactory groove meningiomas: Functional outcome in a series treated microsurgically. Acta Neurochir (Wien). 2006;149(2):109-21. [Google Scholar]
- Ciurea A, Iencean S, Rizea R, Brehar F. Olfactory groove meningiomas: a retrospective study on 59 surgical cases. Neurosurg Rev. 2012;35(2):195-202. [Google Scholar]
- Colli B, Carlotti C, Assirati J, Santos M, Neder L, Santos A. Olfactory groove meningiomas: surgical technique and follow-up review. Arq Neuropsiquiatr. 2007;65(3B):795-9. [Google Scholar]
- Nakamura M, Struck M, Roser F, Vorkapic P, Samii M. Olfactory groove meningiomas: Clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60(5):844-52. [Google Scholar]
- Spektor S, Valarezo J, Fliss D, Gil Z, Cohen J, Goldman J. Olfactory groove meningiomas from neurosurgical and ear, nose, and throat perspectives: Approaches, techniques, and outcomes. Neurosurger. 2005;57(4 Suppl):268-80. [Google Scholar]
- Bitter A, Stavrinou L, Ntoulias G, Petridis A, Dukagjin M, Scholz M. The Role of the Pterional Approach in the Surgical Treatment of Olfactory Groove Meningiomas: A 20-year Experience. J Neurol Surg B Skull Base. 2013;74(2):97-102. [Google Scholar]
- Romani R, Lehecka M, Gaal E, Toninelli S, Celik O, Niemela M. Lateral supraorbital approach applied to olfactory groove meningiomas: Experience with 66 consecutive patients. Neurosurgery. 2009;65(1):39-52. [Google Scholar]
- Welge-Luessen A, Temmel A, Quint C, Moll B, Wolf S, Hummel T. Olfactory function in patients with olfactory groove meningioma. J Neurol Neurosurg Psychiatry. 2001;70(2):218-21. [Google Scholar]
- Gindi SE. Olfactory groove meningioma: surgical techniques and pitfalls. Surg Neurol. 2000;54(6):415-7. [Google Scholar]
- Rubin G, David U, Gornish M, Rappaport Z. Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir (Wien). 1994;129(1-2):26-30. [Google Scholar]
- SP, Fiore P, Levita A, Camera AL, Cambria S. Basal meningiomas. A retrospective study of 139 surgical cases. J Neurosurg Sci. 1999;43(2):107-13. [Google Scholar]
- Romani R, Lehecka M, Gaal E, Toninelli S, Celik O, Niemela M. Lateral supraorbital approach applied to olfactory groove meningiomas: experience with 66 consecutive patients. Neurosurgery. 2009;65(1):39-52. [Google Scholar]
- Schaller C, Rohde V, Hassler W. Microsurgical removal of olfactory groove meningiomas via the pterional approach. Skull Base Surg. 1994;4(4):189-92. [Google Scholar]
- Tomasello F, Angileri F, Grasso G, Granata F, Ponte FD, Alafaci C. Giant olfactory groove meningiomas: extent of frontal lobes damage and long-term outcome after the pterional approach. World Neurosurg. 2011;76(3-4):311-7. [Google Scholar]
- Turazzi S, Cristofori L, Gambin R, Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999;45(4):821-5. [Google Scholar]
- Hentschel S, Demonte F. Olfactory groove meningiomas. Neurosurg Focus. 2003;14(6). [Google Scholar]
- Durante FE. Estirpazione di un tumore endocranico. Arch Atti Soc Ital Chir. 1886;2:252-5. [Google Scholar]
- Cushing H, Eisenhardt L. . The olfactory meningiomas with primary anosmia, in Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results. 1938. [Google Scholar]
- PdA, Tahara A, Almeida A, Simm R, Silva A, Maldaun M. Olfactory groove meningiomas: approaches and complications. J Clin Neurosci. 2009;16(9):1168-73. [Google Scholar]
- Almeida JD, Carvalho F, Filho FVG, Kiehl TR, Koutourousiou M, Su S. Comparison of endoscopic endonasal and bifrontal craniotomy approaches for olfactory groove meningiomas: A matched pair analysis of outcomes and frontal lobe changes on MRI. J Clin Neurosci. 2015;22(11):1733-41. [Google Scholar]
- El-Bahy K. Validity of the frontolateral approach as a minimally invasive corridor for olfactory groove meningiomas. Acta Neurochir (Wien). 2009;151(10):1197-205. [Google Scholar]
- Hassler W, Zentner J. Pterional approach for surgical treatment of olfactory groove meningiomas. Neurosurgery. 1989;25(6):942-5. [Google Scholar]
- Mielke D, Mayfrank L, Psychogios MN, Rohde V. The anterior interhemispheric approach: a safe and effective approach to anterior skull base lesions. Acta Neurochir (Wien). 2014;156(4):689-96. [Google Scholar]
- Park H, Seol H, Nam D, Lee J, Kong D, Kim J. Treatment outcomes after surgical resection of midline anterior skull base meningiomas at a single center. J Clin Neurosci. 2012;19(12):1654-8. [Google Scholar]
- Telera S, Carapella C, Caroli F, Crispo F, Cristalli G, Raus L. Supraorbital keyhole approach for removal of midline anterior cranial fossa meningiomas: A series of 20 consecutive cases. Neurosurg Rev. 2012;35(1):67-83. [Google Scholar]
- Tuna H, Bozkurt M, Ayten M, Erdogan A, Deda H. Olfactory groove meningiomas. J Clin Neurosci. 2005;12(6):664-8. [Google Scholar]
- Zimmer L, Theodosopoulos P. Anterior skull base surgery: open versus endoscopic. Curr Opin Otolaryngol Head Neck Surg. 2009;17(2):75-8. [Google Scholar]
- Kempe L, Vanderark G. Anterior communicating artery aneurysms. Gyrus rectus approach. Neurochirurgia (Stuttg). 1971;14(2):63-70. [Google Scholar]
- Yaşargil M. General Operative Techniques, in Microneurosurgery. . 1984;4:208-33. [Google Scholar]
- Olivecrona H, Urban H. Über Meningeome der Siebbeinplatte. Brun’s Beitr Klin Chir . 1935;161:224-53. [Google Scholar]
- Al-Mefty O, LS, IJ. Tuberculum sella and olfactory groove meningiomas. Surgery of Cranial Base Tumors. 1993. [Google Scholar]
- Babu R, Barton A, Kasoff S. Resection of olfactory groove meningiomas: Technical note revisited. Surg Neurol. 1995;44(6):567-72. [Google Scholar]
- Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53(3):534-42. [Google Scholar]
- Gazzeri R, Galarza M, Gazzeri G. Giant olfactory groove meningioma: Ophthalmological and cognitive outcome after bifrontal microsurgical approach. Acta Neurochir (Wien). 2008;150(11):1117-25. [Google Scholar]
- Avella D, Salpietro FM, Alafaci C, Tomasello F. Giant olfactory meningiomas: the pterional approach and its relevance for minimizing surgical morbidity. Skull Base Surg. 1999;9(1):23-31. [Google Scholar]
- Adegbite A, Khan M, Paine K, Tan L. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58(1):51-6. [Google Scholar]
- Al-Mefty O, Holoubi A, Rifai A, Fox J. Microsurgical removal of suprasellar meningiomas. Neurosurgery. 1985;16(3):364-72. [Google Scholar]
- Black P. Meningiomas. Neurosurgery. 1993;32(4):643-57. [Google Scholar]
- Derome P, Guiot G. Bone problems in meningiomas invading the base of the skull. Clin Neurosurg. 1978;25:435-51. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Mishra VK, Chand VK, Singh N, Gupta A, Singh DK. A comparative evaluation of microsurgical excision of olfactory groove meningioma through unilateral (pterional) vs Bilateral (Bifrontal-transbasal) approach [Internet]. IP Indian J Neurosci. 2023 [cited 2025 Oct 21];9(4):203-208. Available from: https://doi.org/10.18231/j.ijn.2023.040
APA
Mishra, V. K., Chand, V. K., Singh, N., Gupta, A., Singh, D. K. (2023). A comparative evaluation of microsurgical excision of olfactory groove meningioma through unilateral (pterional) vs Bilateral (Bifrontal-transbasal) approach. IP Indian J Neurosci, 9(4), 203-208. https://doi.org/10.18231/j.ijn.2023.040
MLA
Mishra, Vineet Kumar, Chand, Vipin Kumar, Singh, Neha, Gupta, Amit, Singh, Deepak Kumar. "A comparative evaluation of microsurgical excision of olfactory groove meningioma through unilateral (pterional) vs Bilateral (Bifrontal-transbasal) approach." IP Indian J Neurosci, vol. 9, no. 4, 2023, pp. 203-208. https://doi.org/10.18231/j.ijn.2023.040
Chicago
Mishra, V. K., Chand, V. K., Singh, N., Gupta, A., Singh, D. K.. "A comparative evaluation of microsurgical excision of olfactory groove meningioma through unilateral (pterional) vs Bilateral (Bifrontal-transbasal) approach." IP Indian J Neurosci 9, no. 4 (2023): 203-208. https://doi.org/10.18231/j.ijn.2023.040
