- Visibility 87 Views
- Downloads 34 Downloads
- Permissions
- DOI 10.18231/j.ijn.2022.035
-
CrossMark
- Citation
β-oscillations linked by neuronal-spiking in the STN via DBS of parkinson disease: Part – I
- Author Details:
-
V Rama Raju *
Abstract
Typical head-worn brain surface (wired or wireless) EEG electrodes beta (β) band frequencies are amongst 13Hz to 30Hz. However, in the Parkinson`s the high beata frequency is between 20 Hz to 30 Hz. Atypical β-oscillations in BG were linked in the field of Patho physiology of Parkinson disease. 13Hz-30Hz β-oscillations into STN of Parkinson subjects has been verified to distress the spatio-temporal dynamics of high-frequency oscillations (HFOs) typically ranging from 200Hz–500Hz coupled with individual (single) neurons, probably and so hypothetically cooperating the functional litheness (or suppleness) of the motor-circuit. This study experimented the connections through concurrently gathering the field-potentials (i.e.,LFPs) plus single-unit-activity(SUA) from the parallelly connected basal-ganglion circuitry of 15 subjects (PD-patients) in deep-brain-stimulation(DBS) surgical-procedure of bilateral-STN. Stimulus phase-amplitude coupling (PAC) around nuclei was limited to β-phase plus HFO stimulus-amplitude. Coupling was greatest on the abaxial, i.e., dorsal- STN frontier-edge. Findings showed that greater β-HFO-PAC in close proximity (within the vicinity) macro-leads contact which were clinically useful evaluated by continuing un effective-contacts, implying that PAC could prognostic of retort to STN-DBS. Neural-spiking was confined to the shape of 7Hz–30Hz oscillations (/fluctuations in the membranes), then longitudinal-topo-graphy of spike-shape-locking(SSL)/ or spike-phase-locking(SPL) was analogous to PAC. Differences of PAC plus SSL/(SPL) indicated a lack—of spatio-temporal-correlations. β-coupled H.F.O.s and electrical-field protected (locked/fused) neurons have got unique and ideal phase-angles, i.e., signal/waveform-shapes above(+) x-axis and below (-) axis 2D spatio-temporal regions, didn`t transpired in the similar phase of modulating-oscillation. Hence, results yield further help which β-HFO-P.A.C might be key to pathos physiology of Parkinson which advises local electrical field-locked neurons are inadequate alone for the appearance of high frequency β-coupled oscillations.
Introduction
Typical head-worn brain surface (wired or wireless) EEG electrodes beta (β) band frequencies are amongst 13 Hz to 30 Hz. However, in the Parkinson`s the high beata frequency is between 20 Hz to 30 Hz.[1] Atypical β-oscillations in the Basal- Ganglia (BG) were linked in the field of Patho physiology of Parkinson disease(PD). The β-oscillations in sub thalamic nucleus (STN) are decreased in conjunction with voluntary movement,[1] a combined L-Dopa[2] and deep brain stimulation (DBS) study,[3] implied and indicated that the β-oscillations might be anti-kinetic.[4] On the other hand, γ plus high-frequency (200–500Hz; 60 –90Hz) oscillations, i.e., HFOs, that are further prominent in the existence of exogenic-dopamine while DBS is on “ON state, i.e., DBS-ON”,[5], [6] might be pro-kinetic since the movement provokes (or stimulates), i.e., elicits and enhances in phantom (spectral) power in these frequency-bands.[7], [8], [9] However, in what way abnormal (aberrant) activities of these fluctuations advances and indicates to the hypo kinetic motoric features of Parkinson disease is not wholly established.
Some studies indicating that it might not be beta β-fluctuations, as such, intrinsically however instead its impact on neuronal activity implicated in the sphere of motoric processing which cause the failure to choose particular programmes of motoric-activity as well as movement.[10], [11], [12] These connections, conceding the information coding skill or rational springiness of the motoric circuitry, possibly will occur at the point of individual neurons[1], [13] or of neural-net-works.[9], [14], [15], [16] Therefore, β fluctuations attune of single-unit (SU) spiking—activity (SA), i.e., single unit activity (SUA) or single neuron activity (SNA).[17], [18], [19] or else pro kinetic higher frequency fluctuations[9] in the region of the subthalamus might well establish the patho-logic mechanism which yields to the detected hypo kinetic symptoms within the Parkinson subjects (patients). Microelectrode recording (MER) signals acquired in the course of DBS surgical-procedure suggest a chance to precisely investigate these connections in the motoric-circuit of Parkinson.
We gathered the field potentials, i.e., LFPs around the nuclei, i.e., STN to observe the spatio-magnitude of cross-correlation through transformed frequency domain Fast Fourier Transform (FFT) frequency connections amongst β-fluctuations as well as high frequency oscillations. Results showed that the connections were extremely significant and very valuable at the d o r s a l sub thalamic nuclei border also, the intensity of these interfaces were linked to the experimental value of DBS stimuli. Single and multi-unit activity (SUA and MUA) of STN neurons were acquired with support vector machine (SVM) based MER system asynchronously (parallelly) and the main purpose is to study the areal point of locked electrical field potentials movement and activity. We notice that these connections or interfaces were highest at the d o r s a l STN border. We largely observed the co-existence and co-incidence incidence of FFT based cross-correlation frequency also local electrical-field-spikes contacts and found no evidence of a causal relationship.
Aims and Objectives
To study the contacts and connections of the stimulating electrodes implanted by DBS surgical procedure.
To acquire the concurrent spike recordings of the electrically local field potentials and also single and multi-unit activities of the parallelly connected basal ganglion circuitry in the nervous system, i.e., MER with bilateral sub thalamic nucleus deep brain stimulation in Parkinson`s. Phase of the amplitude through the stimulus intensity by the micro and macro electrodes by microelectrodes stimulations and macrostimulations coupling in the sub thalamic nuclei which was precise to β-phase plus high frequency oscillations stimulus amplitudes, followed by the coupling at the abaxial sub thalamic nucleus border.
Materials and Methods
The data-analyses were achieved by applying the custom-built MATLAB (offline) software-scripts (Math Works). We applied a two-tailed ‘t-test’ to evaluate for statistical significancy together with the implementation of a chi-square test and degree of freedom so as to say whether statistically significant on the validation of chi-square , and except if not (or-else) remarked.
Clinical demography and characteristics of Parkinson subjects and DBS operational procedure
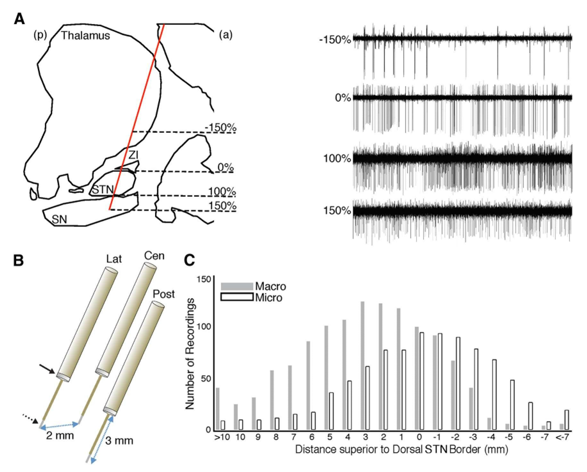
We captured intraoperative recordings in 12 Parkinson`s diseased subjects, i.e., patients with a male female ratio 7:5 (7 male and 5male) through advanced idiopathic Parkinson disease PD undergoing minimally invasive deep brain stimulation surgery (i.e., DBS procedural operational surgery) of the bi lateral subthalamic nucleus - S T N. Every Parkinson subject based on United Kingdom (UK) brain bank criterion for invasive (minimal) operating dealing of the Parkinson`s diseased patients as well as endured and experienced the DBS-surgery followed by immediate and instantaneously L-Dopa (levodopa) dopaminergic-medication removal, typically 12hours since the last-dose). Each and every signal-recording, i.e., MER signal recording was gathered when the Parkinson subjects were conscious, widely-awakened and during their rest, as well as examined for carefully and hence attentively observance. Following, the sub thalamic nucleus was confined structurally/anatomically on pre op magnetic resonance-imaging as well as by intra operatively its stimulating-firing-patterns (SFPs) and also related activity, Figure 1. A. right-hand side. The x and y plane, i.e., x and y axes co -ordinates of the pre-operative target, referenced to the midcommissural point, were x=11.1±0.2 (mean±SEM), y= —3.5±0.5, z=4.9±0.3mm for the left-hemi-sphere (i.e., left-side brain), and x= —11.5±0.21, y= —3.6±0.5, z=5.01± 0.3mm for the right-hemi-sphere (i.e., right-side brain). All the Parkinson subjects (patients) were embedded the micro and macro electrodes with Medtronic (USA maker) deep brain stimulations—DBS lead-models:3389 (1.5mm contacts with center-to-center distances of 2mm.
Signal recording
The intra operative micro electrode signal recordings by applying the directing or pointing electrodes encompassing a couple of macro lead-electrode (for macro stimulations) and micro-electrode recording contacts, wherein the macro electrode interaction was 3.5mm superior to the micro electrode stimulating contact. Equal to the 3 aimed-electrodes, i.e., targeting-electrodes were parallelly forward-thinking to the pre op target throughout and all through every MER recording session. The just acquired raw signals—waveforms were sampled at 1.5kHz-frequency and 24kHz-frequency as of macro electrodes as well as micro electrodes, correspondingly, plus accumulated by using the micro α-ω(alpha-omega)guide pro-data acquisition-system. Micro electrode signal (data) since on or after the first 4 PD subjects/ patients were filtered with high-pass filter ~>=300Hz, whose frequency is over≥300Hz through a acausal skull-opening-stage filter (head). Per se, intrinsically, we discarded the micro electrodes data(i.e., MER-signals data) on or after the first 4 Parkinson`s subjects from our inferences that we have drawn. For later and successive PD patients, the raw micro electrode data (MER signals) were gathered by applying a broad band frequency ranging from 1.5Hz to 3kHz acausal skull-cranium (head) opened-stage filter. The local field electrical potentials were elicited through the band pass filters and macro-lead-electrode (MLR) and micro electrode (MER) signals amid 1Hz to 500Hz, and with a 50Hz notch-filter so as to uproot the noise from electrical appliances instrument mains lines, acoustics, etc. (according to Indian English Channel).
All the local field electrical potentials were sampled down(down—sampled) to 0.5kHz and referenced macro lead-electrodes and micro electrode were signals by applying a common average of every signal acquired with the macro lead electrodes and micro electrodes signals/waveforms concurrently. We encountered some artifacts like horse-jumps in the waveforms which were also detected in local field electrical potentials beta-oscillations while every acquiring signal during the z scored broad band (wide band) frequencies ranging from 20Hertz to 300Hertz and the 26 S Ds power exceeded from the electrical baseline, i.e., the zero line). For every MER signal acquisition(MER with STN-DBS), the extended portion set free of horse-jump noise-artifacts(distortions, blur, etc.) as well as biological-amplifier (op-amp instrumentation but the impedance was increased up to 300 Meg Ohms were inferred or deduced, eliminating those signal-acquisitions through artifact-free segments that were less than 5Seconds.
Results
The intra operative micro lead electrode signals were acquired through macro stimulation (i.e., macrosimulation) and micro electrodes recording (MER) was performed through micro-stimulation and both the techniques through deep brain stimulation, i.e., DBS during the bilateral STN DBS surgical procedural process. We took intra op recordings during the deep brain surgery of 12 advanced idiopathic Parkinson`s disease bilateral sub thalamic nucleus deep brain stimulation (STN—DBS) patients male ratio:58.1±2.6 years-old). The disease duration was 13.8± 1.9/ mean age at the onset years, through the pre op UPDRS scale (part/stage-III) score of 18.9 ±1.6 during the DBS is in the ON state (DBS ON) as well as 38.5±2.4 during the DBS in the OFF state (i.e., DBS OFF). As the directly pointing the electrodes were innovative progressively in the direction of the pre-op-target, see[Figure 1] . (A) and (B), we recognized the sub thalamic nuclei s p i k i n g - a c t i v i t y for 3.75±0.19mm in to the 66 of the complete 115-trajectories.
Conclusions
Our findings indicate that the longitudinal topography of β-H.F.O and 8Hz–30Hz in relatively to the exact and specific electro physiological (i.e., electrophysiological signals acquired through MER system for the signature patterns of STN) limits of nuclei. Protuberant and protruding and projecting β-fluctuations (i.e., oscillations of local field electrical β-potentials) occur in neural biomarkers acquisitions of the sub thalamic nucleus Parkinson`s during the DBS is in OFF state (i.e., DBS-OFF) and these oscillations have an effect on the progressive and temporal dynamics of HFOs and single neurons. This study enlightened on the MER with bilateral STN DBS in advanced idiopathic Parkinson`s disease patients. Further experimental analysis will be discussed in our next article with more findings
Source of Funding
No funding or grant support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Hernán M, Fernando C, Otárola J, Rojas R, Alarcón O, Cañete L. EEG Beta band frequency domain evaluation for assessing stress and anxiety in resting, eyes closed, basal conditions. Procedia Computer Sci. 2019;162:974-81. [Google Scholar] [Crossref]
- Levy R, Ashby P, Hutchison W, Lang A, Lozano A, Dostrovsky J. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002;125(6):1196-209. [Google Scholar] [Crossref]
- Eusebio A, Thevathasan W. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatr. 2011;82:569-73. [Google Scholar]
- Andrew I, Vanegas N, Lungu C, Zaghloul K. Beta-Coupled High-Frequency Activity and Beta-Locked Neuronal Spiking in the Subthalamic Nucleus of Parkinson's Disease. J Neurosci. 2014;34(38):12816-27. [Google Scholar] [Crossref]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali, P, Lazzaro VD. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21(3):1033-8. [Google Scholar] [Crossref]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E. 300-Hz subthalamic oscillations in Parkin- son’s disease. Brain. 2003;126(10):2153-63. [Google Scholar] [Crossref]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Lazzaro V. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125(6):1235-46. [Google Scholar] [Crossref]
- Androulidakis AG, Kühn A, Chen C, Blomstedt P, Kempf F, Kupsch A. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson’s disease. Brain. 2007;130(2):457-68. [Google Scholar] [Crossref]
- López-Azcárate J, Tainta M, Rodríguez-Oroz M, Valencia M, González R, Guridi J. Coupling be- tween beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 2010;30(19):6667-77. [Google Scholar] [Crossref]
- JM. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381-425. [Google Scholar] [Crossref]
- Quiroga-Varela A, Walters J, Brazhnik E, Marin C, Obeso J. What basal ganglia changes underlie the parkinsonian state? The significance of neuronal oscillatory activity. Neurobiol Dis. 2013;58:242-8. [Google Scholar] [Crossref]
- Brittain JS, Brown P. Oscillations and the basal ganglia: motor control and beyond. Neuroimage. 2014;85(2):637-47. [Google Scholar] [Crossref]
- Levy R, Hutchison W, Lozano A, Dostrovsky J. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20(20):7766-75. [Google Scholar] [Crossref]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi A, Pellegrini M. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurology. 2004;189(2):369-79. [Google Scholar] [Crossref]
- Marceglia S, Foffani G, Bianchi A, Baselli G, Tamma F, Egidi M. Dopamine-dependent non-linear correlation between subtha- lamic rhythms in Parkinson’s disease. J Physiol. 2006;571(3):579-91. [Google Scholar] [Crossref]
- Ku¨hn A. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp Neurol. 2005;194(1):212-20. [Google Scholar] [Crossref]
- Weinberger M, Mahant N, Hutchison W, Lozano A, Moro E, Hodaie M. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol. 2006;96(6):3248-56. [Google Scholar] [Crossref]
- Weinberger M, Hutchison W, Lozano A, Hodaie M, Dostrovsky J. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson’s disease patients. J Neurophysiol. 2009;101(2):789-802. [Google Scholar] [Crossref]
- Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by parkinsonian neuronal oscillations and synchrony. Brain. 2008;131(12):3395-409. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
Raju VR. β-oscillations linked by neuronal-spiking in the STN via DBS of parkinson disease: Part – I [Internet]. IP Indian J Neurosci. 2022 [cited 2025 Sep 23];8(3):167-171. Available from: https://doi.org/10.18231/j.ijn.2022.035
APA
Raju, V. R. (2022). β-oscillations linked by neuronal-spiking in the STN via DBS of parkinson disease: Part – I. IP Indian J Neurosci, 8(3), 167-171. https://doi.org/10.18231/j.ijn.2022.035
MLA
Raju, V Rama. "β-oscillations linked by neuronal-spiking in the STN via DBS of parkinson disease: Part – I." IP Indian J Neurosci, vol. 8, no. 3, 2022, pp. 167-171. https://doi.org/10.18231/j.ijn.2022.035
Chicago
Raju, V. R.. "β-oscillations linked by neuronal-spiking in the STN via DBS of parkinson disease: Part – I." IP Indian J Neurosci 8, no. 3 (2022): 167-171. https://doi.org/10.18231/j.ijn.2022.035
